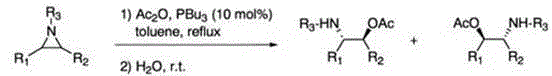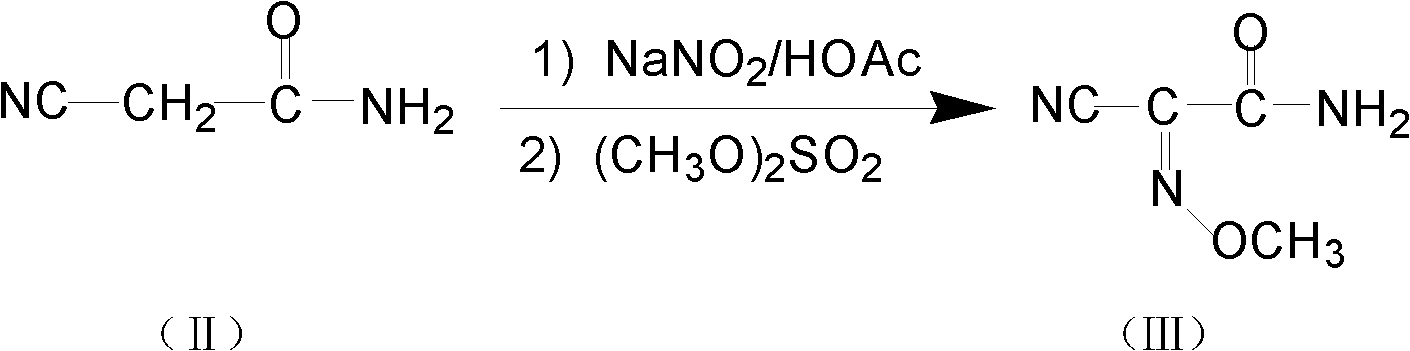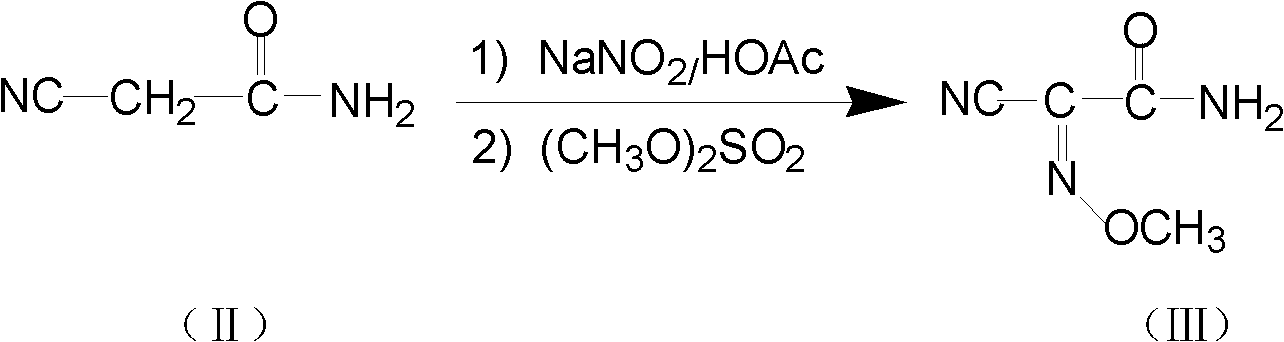Patents
Literature
342 results about "Tosyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
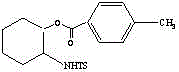
A toluenesulfonyl (shortened tosyl, abbreviated Ts or Tos) group is CH₃C₆H₄SO₂. This group is usually derived from the compound tosyl chloride, CH₃C₆H₄SO₂Cl (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid. The para orientation illustrated (p-toluenesulfonyl) is most common, and by convention tosyl refers to the p-toluenesulfonyl group.
17-hydroxy c27 steroid compound and its synthesis and use
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
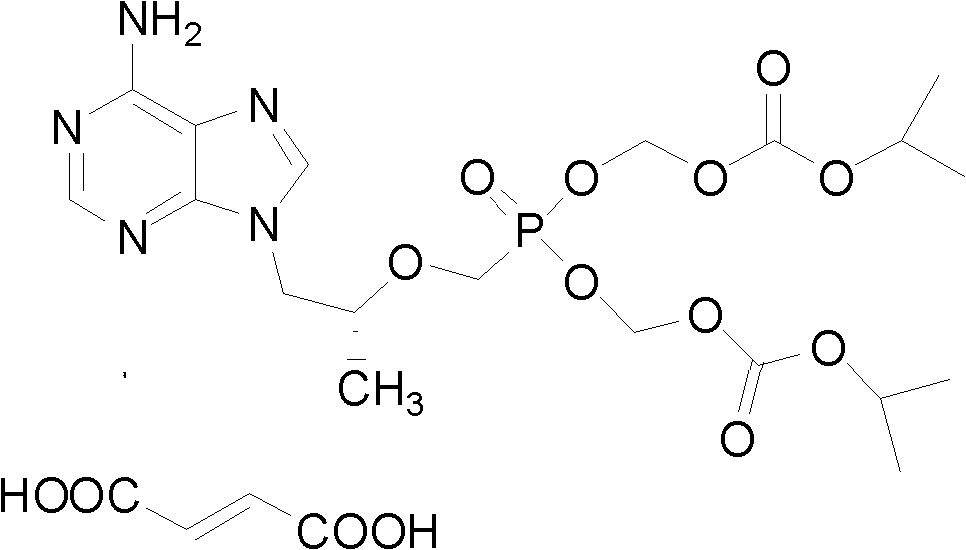
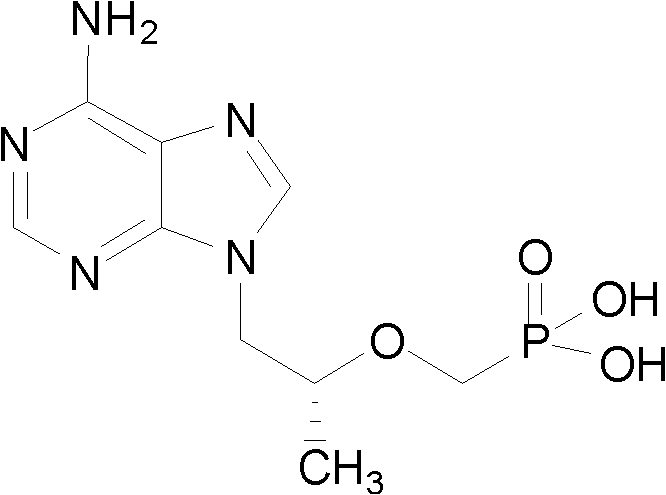
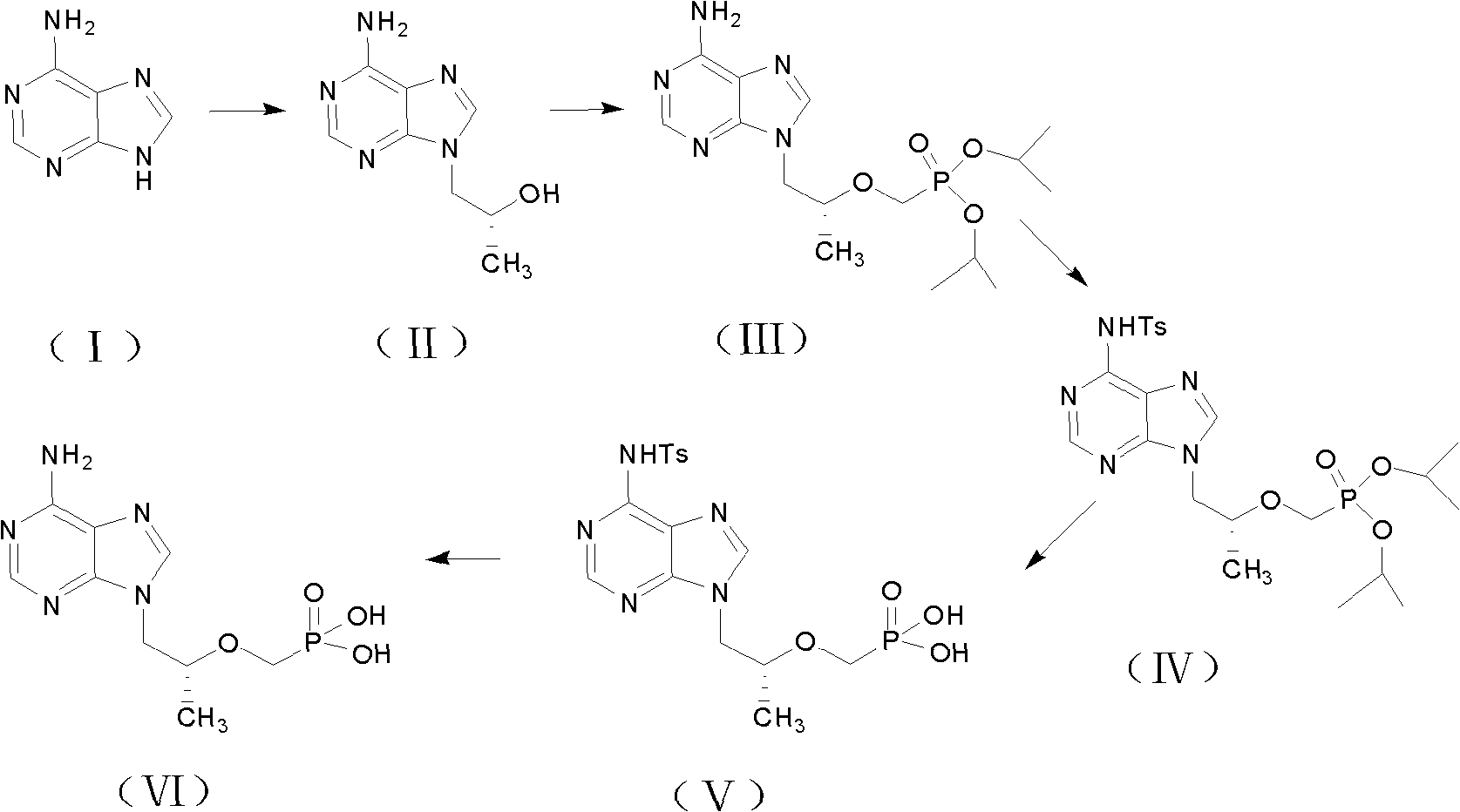
Preparation method for tenofovir
ActiveCN102060876AReduce energy consumptionImprove securityGroup 5/15 element organic compoundsBenzeneAlcohol
The invention discloses a preparation method for tenofovir disoproxil fumarate, which comprises the following steps of: A, performing condensation reaction adenine and (R)-propylene carbonate which serve as raw materials to generate (R)-9-(2-hydroxypropyl) adenine; B, performing condensation reaction on the (R)-9-(2-hydroxypropyl) adenine and p-methylphenyl mesyloxy diethyl phosphonate under the catalysis of potassium alcoholate to prepare (R)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine; C, reacting the (R)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine obtained by the step B with para-toluenesulfonate acyl chloride to protect an amino group at bit four to prepare (R)-4-(p-toluenesulfonyl)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine; D, hydrolyzing the (R)-4-(p-toluenesulfonyl)-9-[2-(diethyl phosphonyl methoxy) propyl] adenine obtained by the step C under a strong acid condition to obtain (R)-4-(p-toluenesulfonyl)-9-[2-(dihydroxy phosphonyl methoxy) propyl] adenine; and E, reacting the (R)-4-(p-toluenesulfonyl)-9-[2-(dihydroxy phosphonyl methoxy) propyl] adenine obtained by the step D with mercapto-benzene under a weak alkaline condition to remove a para-toluenesulfonate group to obtain the tenofovir. The invention aims to provide the preparation method for the tenofovir, which is low in cost, safe in process and good in product quality, and is suitable for industrialization.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
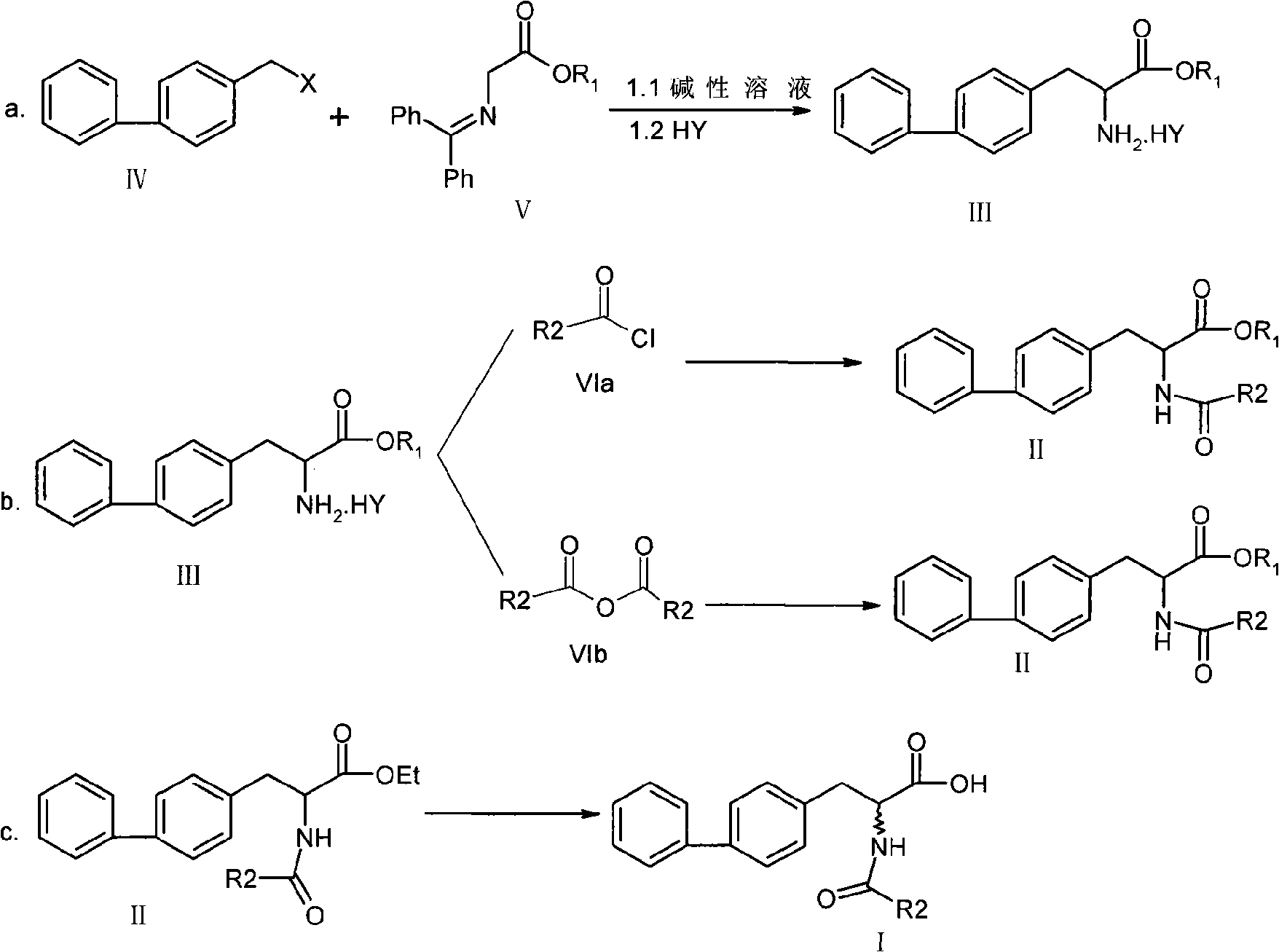
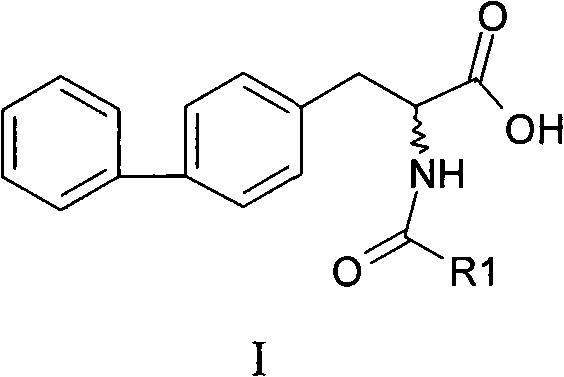
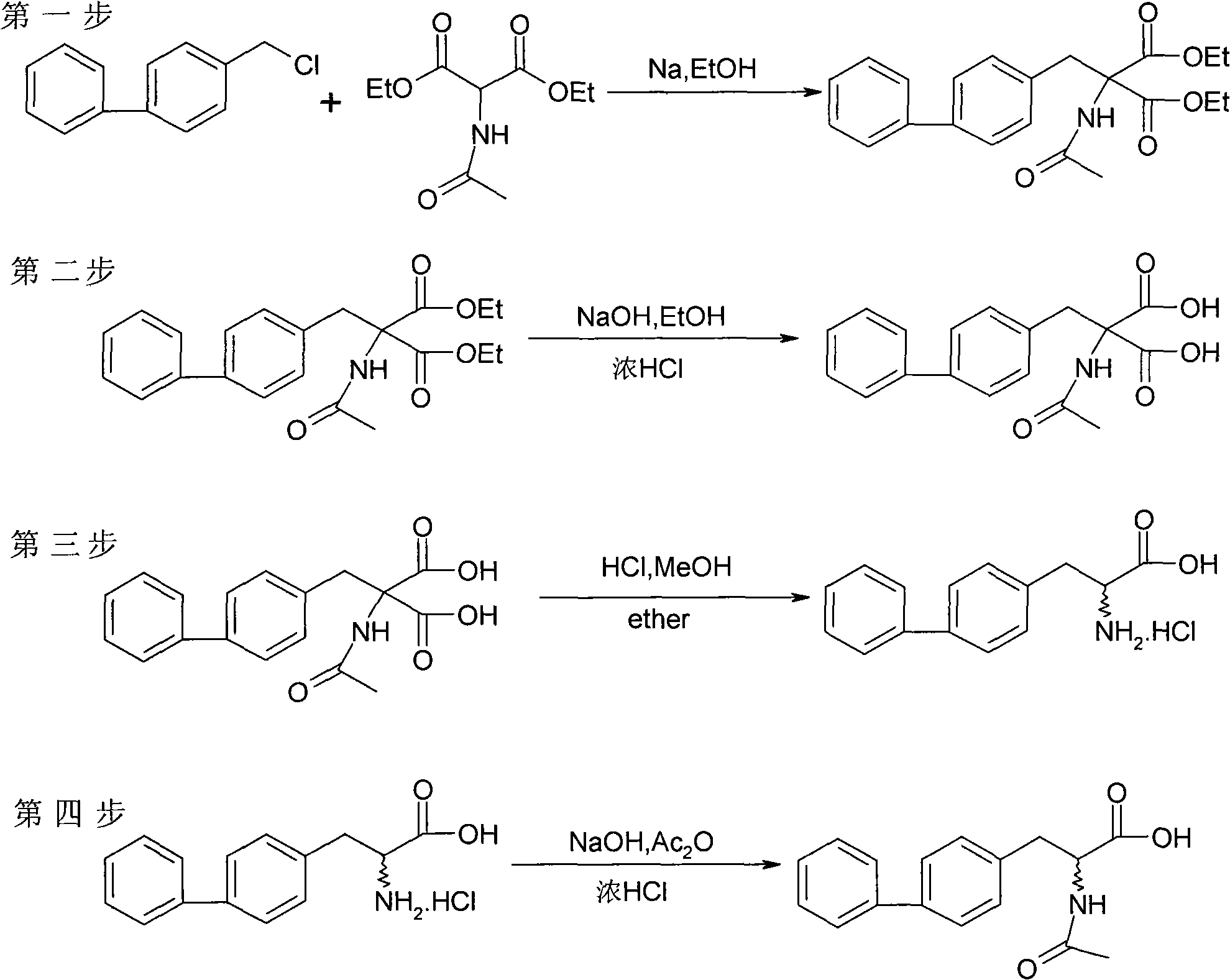
Chemical synthesis method of 2-acylamino-3-biphenyl propionic acid
ActiveCN101555211AHigh selectivityHigh purityOrganic compound preparationCarboxylic acid amides preparationChemical synthesisPropanoic acid
The invention relates to a chemical synthesis method of a 2-acylamino-3-biphenyl propionic acid, and the reaction equation is shown on the right, wherein X is chlorine, bromine, iodine, methylsulfonyl or tosyl; R1 is methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl or benzyl; HY is hydrochloric acid, formic acid, acetic acid, citric acid, oxalic acid, tartaric acid, sulfuric acid, nitric acid or phosphoric acid; R2 is methyl, ethyl, propyl, isopropyl, benzyl or 4-chlorobenzyl. Preferably, the R1 prefers to ethyl or isobutyl; HY prefers to hydrochloric acid; and R2 prefers to ethyl. The invention has short route, simple operation, high reagent selectivity, low production cost, high production purity and high yield, is suitable for large-scale industrial production, and has larger economic and social benefits.
Owner:浙江瑞博制药有限公司
Preparation method of antidepressant drug-vilazodone
InactiveCN103304547AReduce usageReaction raw materials are readily availableOrganic chemistryCarboxylic acidMethyl group
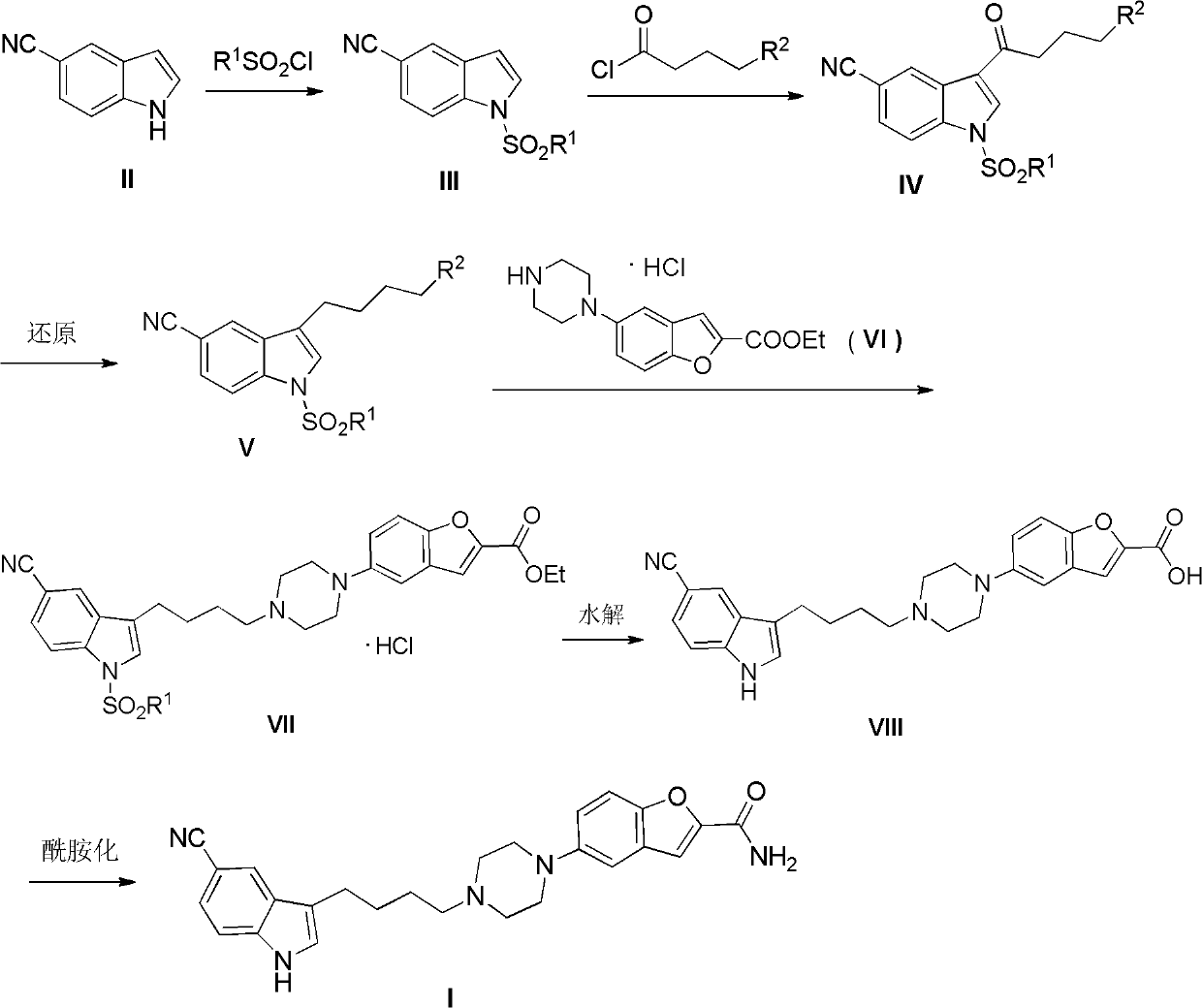
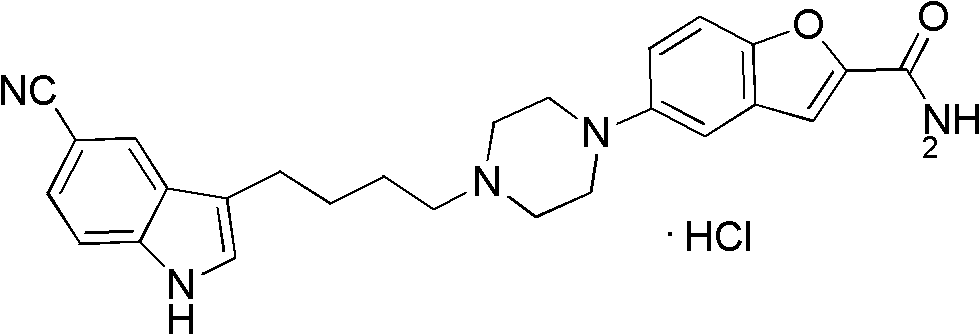
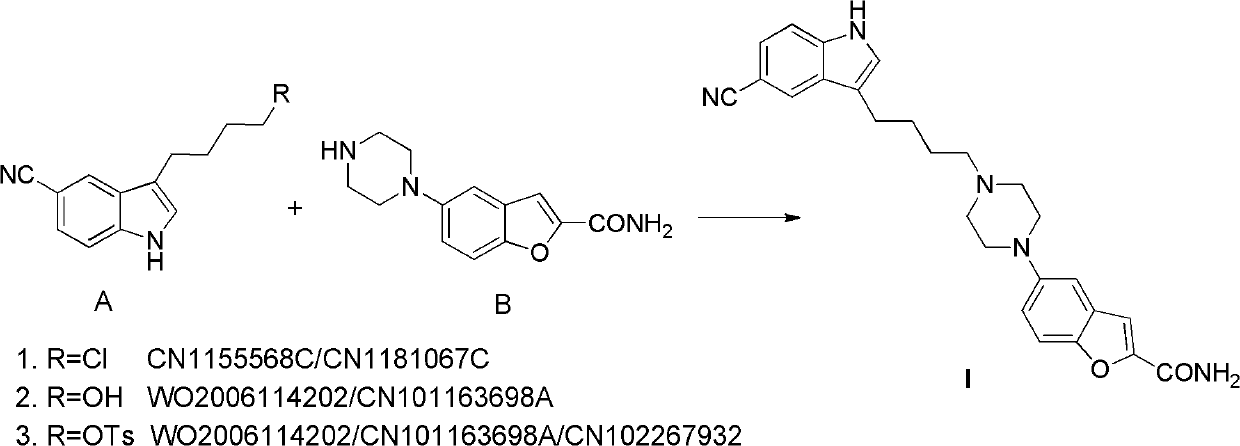
The invention relates to the field of drug synthesis, and in particular relates to a preparation method of an antidepressant drug-vilazodone. The preparation method is characterized by comprising the following steps of: protecting a 1-location nitrogen atom of 5-cyano benzopyrrole used as an initial raw material through utilizing toluenesulfonyl, carrying out 3-location Friedel-Carfts acylation, and selectively reducing carbonyl into methylidyne so as to obtain 1-tosyl-3-(4-chlorobutyl)-5-cyano benzopyrrole; reacting the 1-tosyl-3-(4-chlorobutyl)-5-cyano benzopyrrole with 5-(piperazine-1-radical) benzofuran-2-nonanoic acid-ethyl ester hydrochloride, hydrolyzing through one step, and removing tosyl and ethyl at the same time so as to obtain 5-(4-(4-(5-cyan-1H-benzopyrrole-3-radical) butyl) piperazine-1-radical) benzofuran-2-carboxylic acid; finally, amidating so as to obtain vilazodone, and salifying vilazodone with hydrogen chloride so as to obtain hydrochloric acid vilazodone. The method can be used for overcoming a plurality of defects of a conventional synthetic method, and has the advantages of easiness in raw material acquisition, high yield, good selectivity, convenience in operation, suitability for industrial production and high application value.
Owner:CHINA PHARM UNIV
Method for producing influenza viruses in large scale by using bioreactor
The invention provides a method for producing influenza viruses in large scale by using a bioreactor, which comprises the following steps of: (1) inoculating passage cell suspension into a vector tank, and setting the parameters of the bioreactor to perform a cell adsorption and culture process, wherein a serum culture medium is used in the cell adsorption and culture process; (2) when cells are grown to proper density, rinsing the cells to remove serum components, inoculating influenza virus liquid into the vector tank, and setting the parameters of the bioreactor to perform a virus adsorption and propagation process, wherein a serum-free culture medium containing TPCK (tosyl-L-phenylalanine chloromethyl ketone) pancreatin is used in the virus adsorption and propagation process; and (3) harvesting the virus liquid. The method has the advantage that: the high-titer virus liquid can be obtained by adopting the tide type bioreactor and using the passage cells for propagating the influenza viruses. The method overcomes the defects of the traditional transfer bottle culture and other bioreactors, and can continuously produce high-titer viruses in large scale.
Owner:PU LIKE BIO ENG
Genipin derivative and application thereof
ActiveCN102993158AShorten the overall cycleLow costOrganic active ingredientsOrganic chemistryArylMedicine use
The invention belongs to the field of preparation of new compounds and in particular provides a genipin derivative as well as a preparation method and an application thereof. The genipin derivative and pharmaceutically acceptable salt thereof are shown as a general formula I (described in the specification), wherein R1 is one selected from C1-6 alkyl or alkyl amino, C1-20 aryl, C1-20 aromatic amino and C1-6 heterocyclic amino; R2 is one selected from C1-6 alkyl, C1-20 aryl, C1-6 heterocycle and an amino group; R3 is one selected from C1-6 alkyl, C1-20 aryl, C1-6 heterocycle, methylsulfonyl, p-tosyl group and -COR4, and R4 is one selected from C1-6 alkyl, C1-20 aryl, C1-6 heterocycle and the amino group. The derivative provided by the invention has application value in preparation of medicine used for treating diabetes mellitus II.
Owner:HERBALIFE LEISHUO HUNAN NATURAL PROD
Preparation method and application of modified cyclodextrin polymer hydrogel
ActiveCN108276589APromote degradationReduce manufacturing costOther chemical processesWater contaminantsThioureaPhenol
The invention relates to the technical field of sewage treatment, provides a preparation method and application of cyclodextrin polymer hydrogel, and aims at solving the problems that the conventionalhydrogel is capable of treating single pollutants, and is low in efficiency and small in application range. The preparation method is characterized in that beta-cyclodextrin is used as the raw material and is subjected to selective sulfonylation through toluene sulfochloride to obtain toluenesulfonyl-beta-cyclodextrin; then the toluenesulfonyl-beta-cyclodextrin is subjected to thiourea substituting to obtain hydrosulphonyl grafted beta-cyclodextrin; finally, the hydrosulphonyl grafted beta-cyclodextrin is processed according to a suspension emulsion polymerizing method to obtain the novel two-functional polymer hydrogel. The hydrogel prepared according to the method is applicable to a complex sewage system integrating phenol and heavy metal ions. The hydrogel is capable of efficiently synchronously adsorbing two pollutants including phenol and the heavy metal ions, and is wide in application range and high in adsorption efficiency.
Owner:ZHEJIANG FORESTRY UNIVERSITY
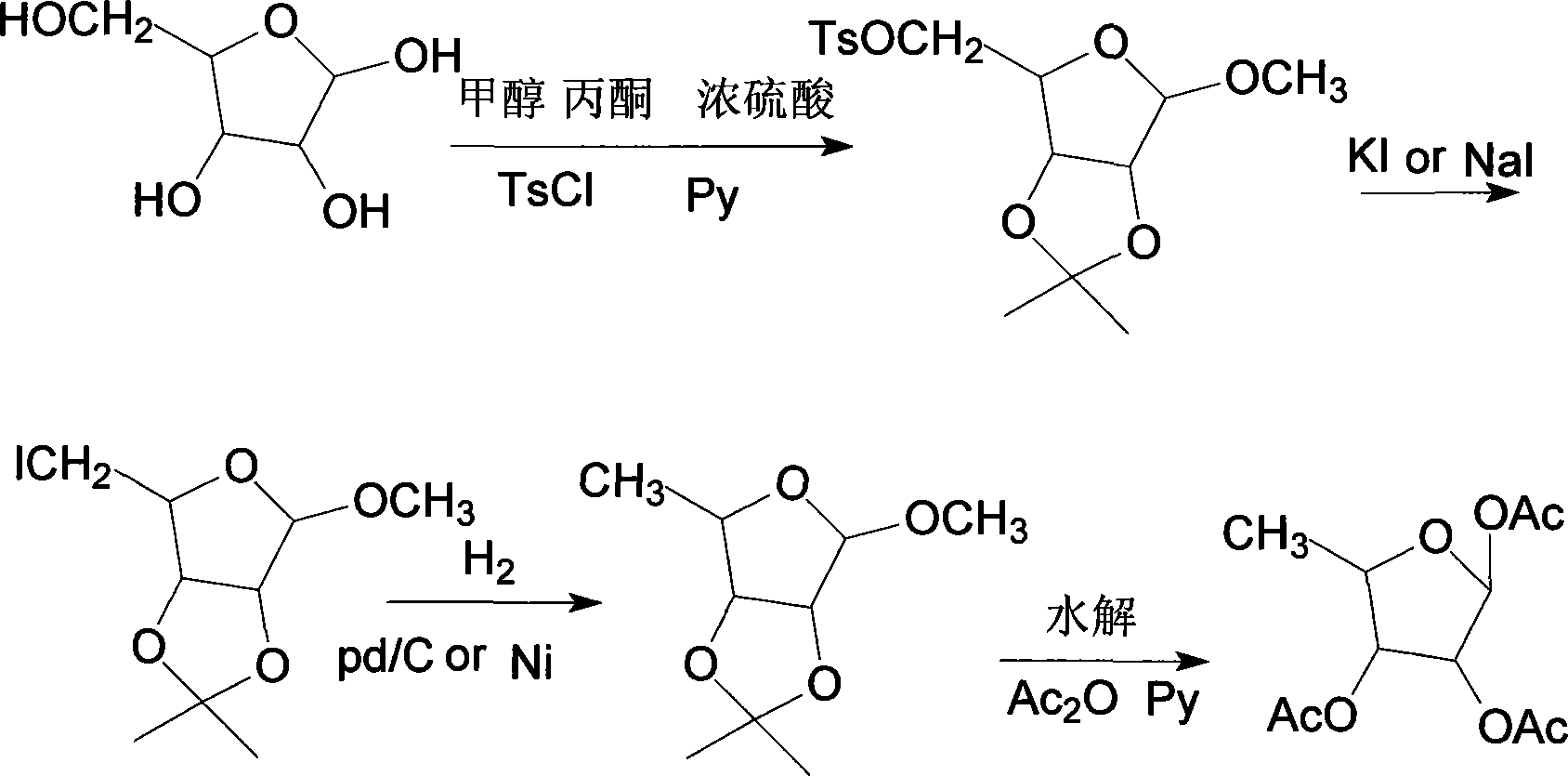
Method for synthesizing 1,2,3-O-tri-acetyl-5-deoxidation-D-ribose
InactiveCN101182342AEasy to operateMild reaction conditionsEsterified saccharide compoundsSugar derivativesFuranSynthesis methods
The invention discloses a synthesis method of 1, 2, 3-O-tri-acetyl-5-deoxy-D-ribose. D-ribose is used as a starting raw material for methyl glycosidation, propylidene and esterification (one-pot method) to obtain 2, 3-O-isopropylidene-5-O-p-tolylsulfonyl-D-furan methyl glycoside which is reduced, hydrolyzed and acetylated to obtain the target product 1, 2, 3-O-tri-acetyl-5-deoxy-D-ribose. The synthesis technology is simple and convenient; the reaction condition is gentle; the raw material is cheap and can be obtained easily; the cost is low; the invention causes no environmental pollution; the total yield is high, and the total yield of the four steps is 37.8 percent; the invention is quite applicable to the industrial production; the product melting point is 62 DEG C to 64 DEG C.
Owner:HEFEI UNIV
Method for preparing quizalofop-p-ethyl
InactiveCN101333194AAvoid it happening againAlleviate environmental pressureOrganic chemistryQuinoxalineEthyl propiolate
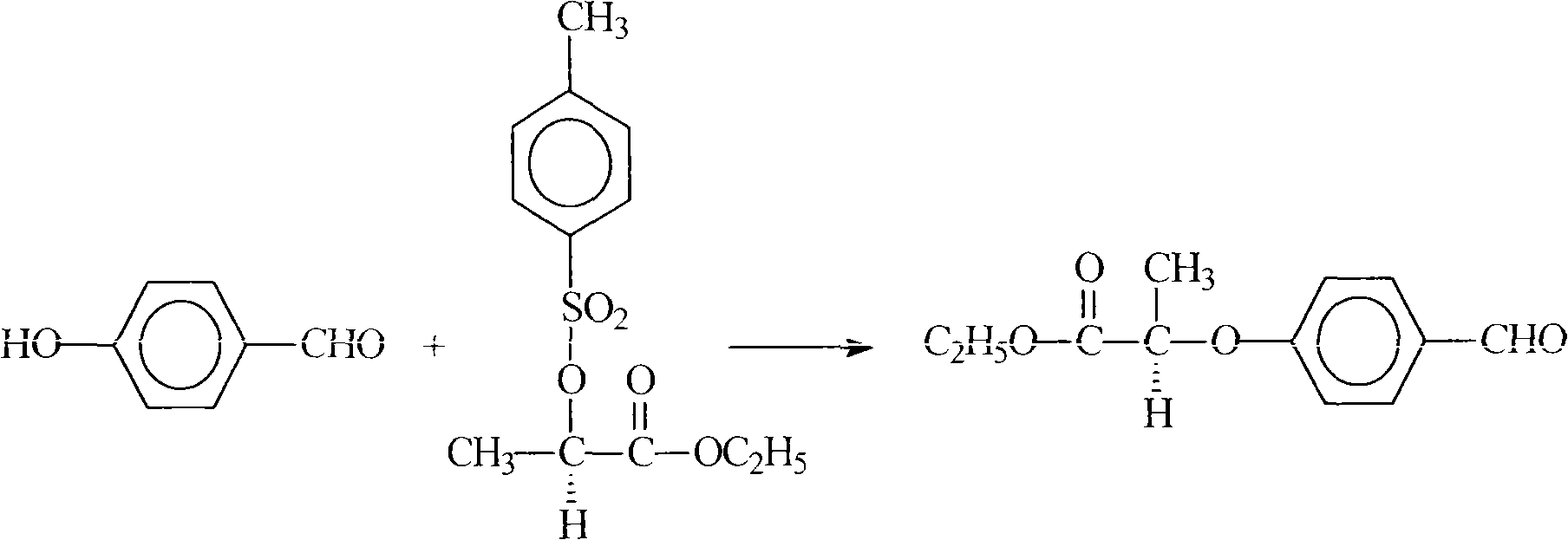
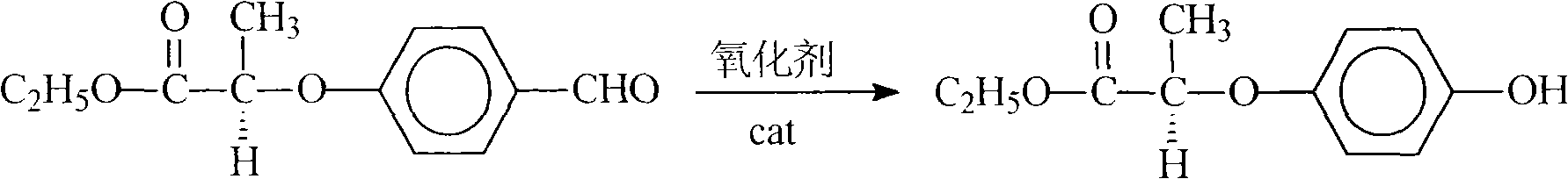
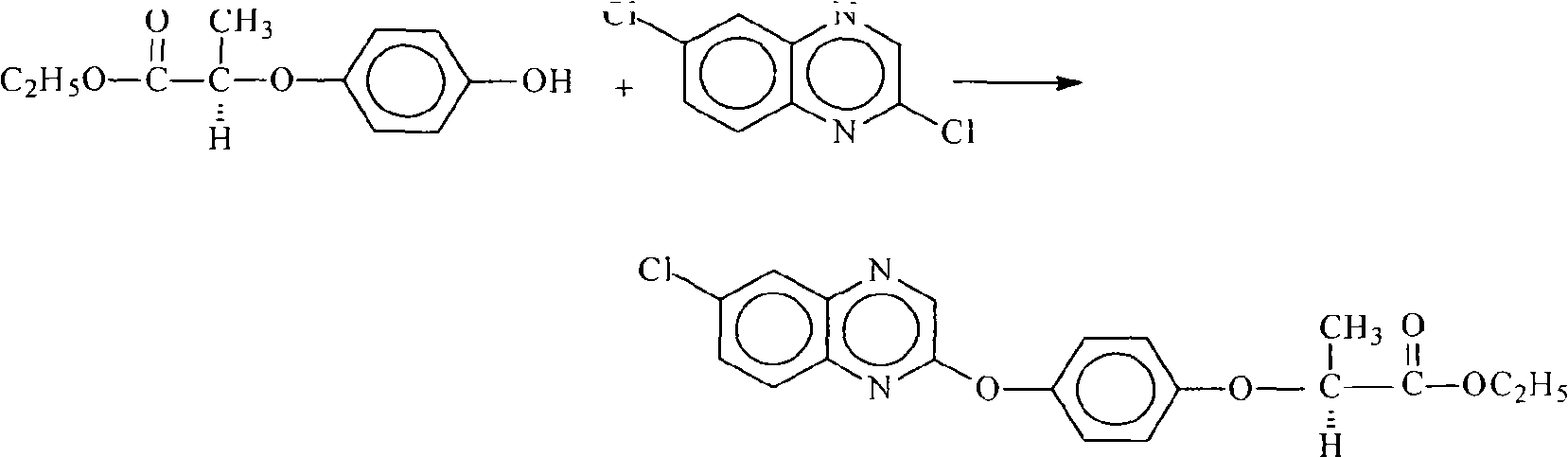
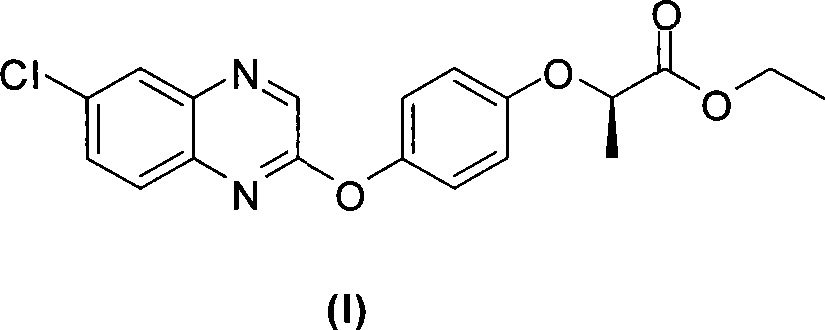
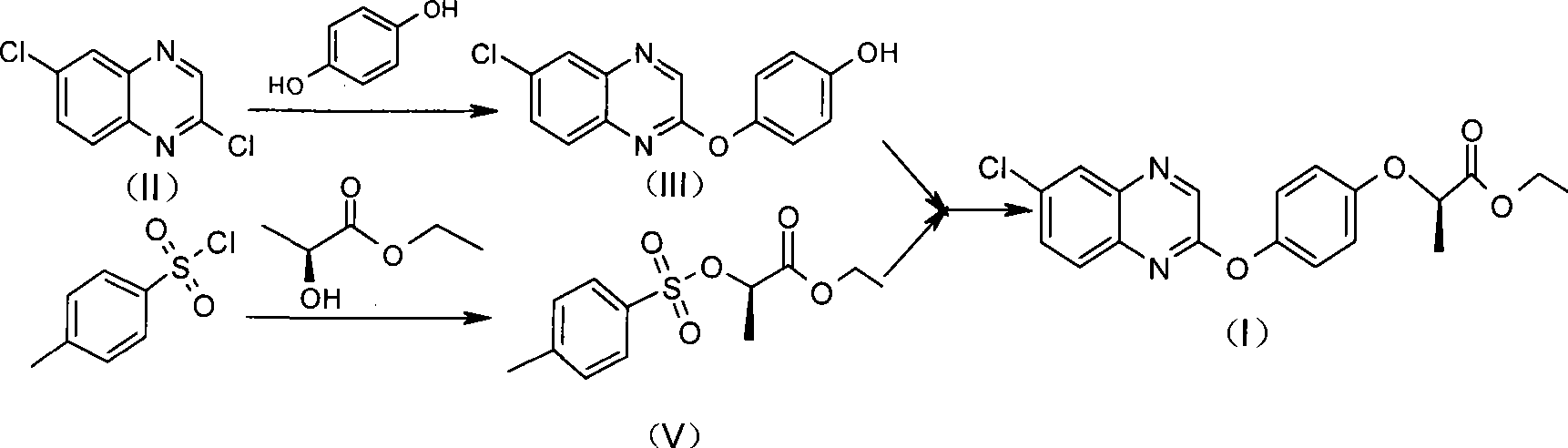
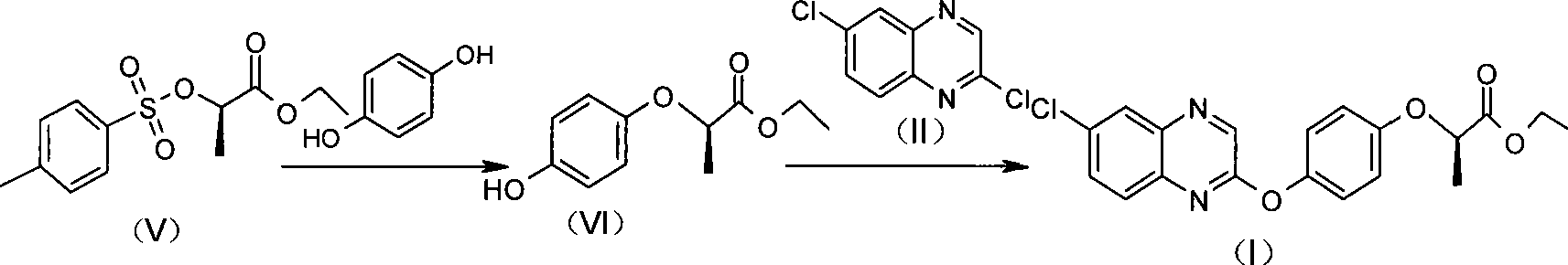
The invention relates to a preparation method for quizalofop-p-ethyl, in particular to a method which uses p-hydroxybenzaldehyde to replace the raw material hydroquinone used in the traditional technology and react with S (-)-N-tosyl ethyl lactate to prepare R (+)-2-(p-oxy) propionate which is then reacted with 2,6- dichloro-quinoxaline to prepare high-purity quizalofop-p-ethyl through condensation.
Owner:SHANDONG CHAMBROAD HLDG GRP CO LTD
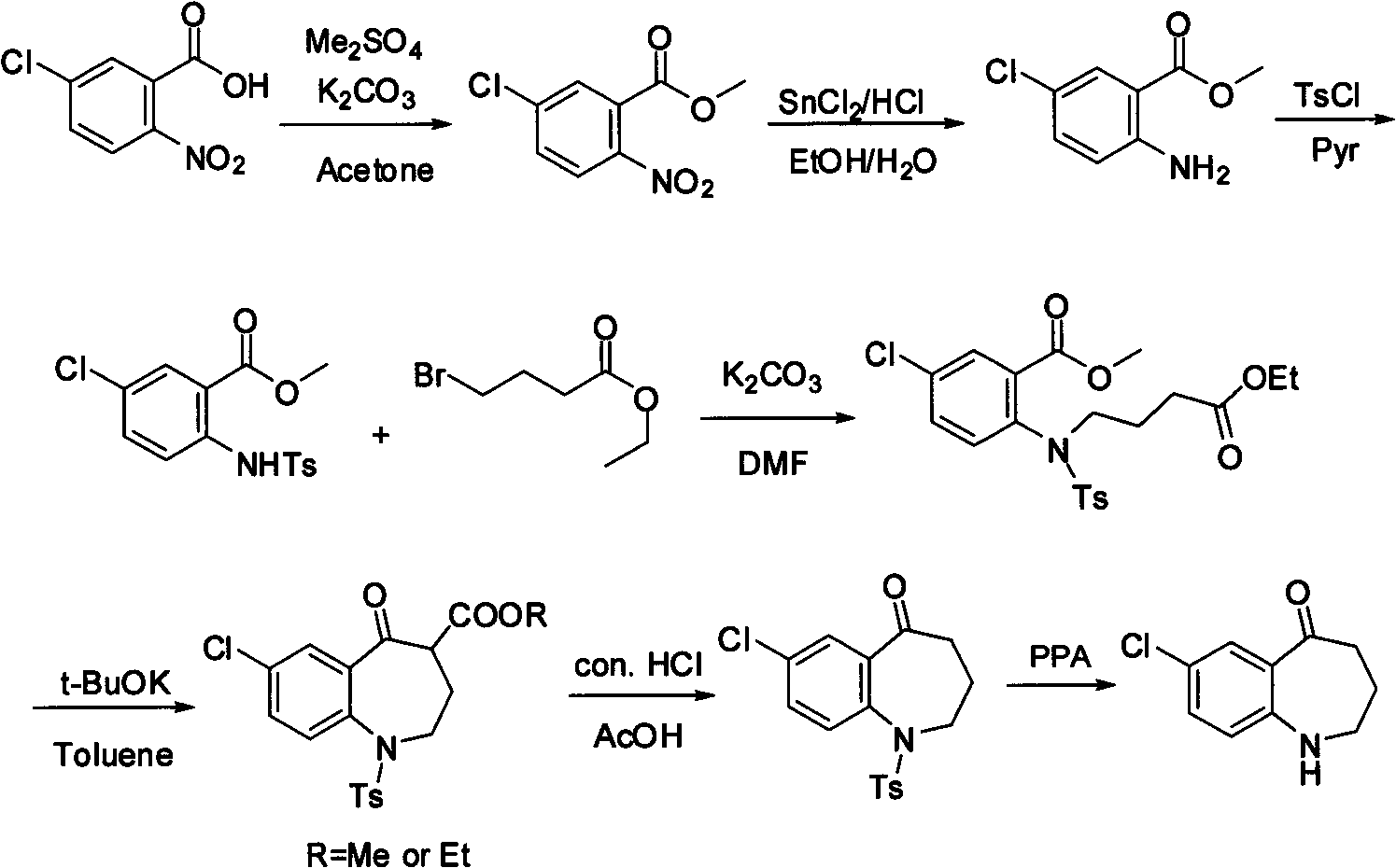
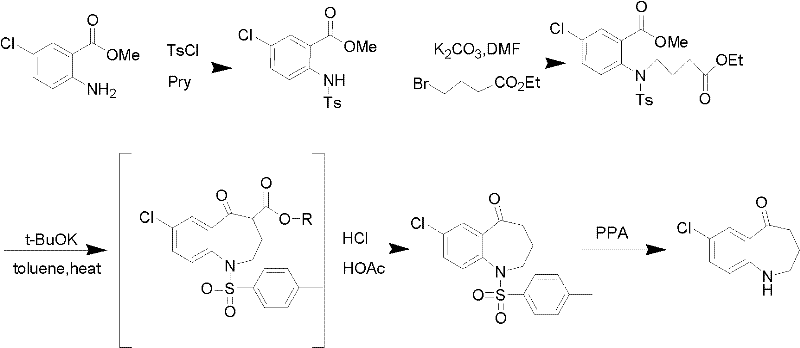
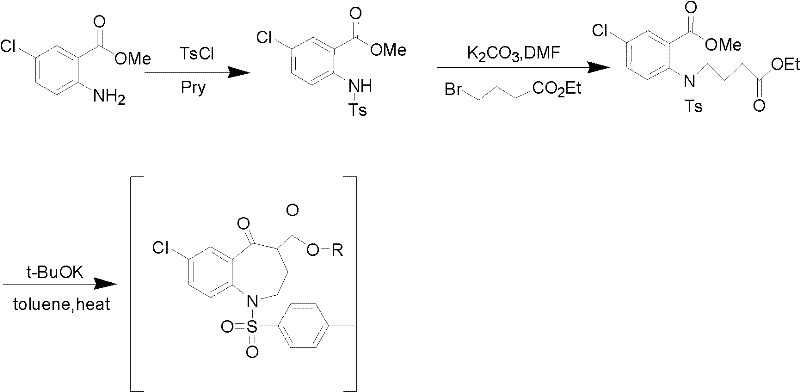
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone
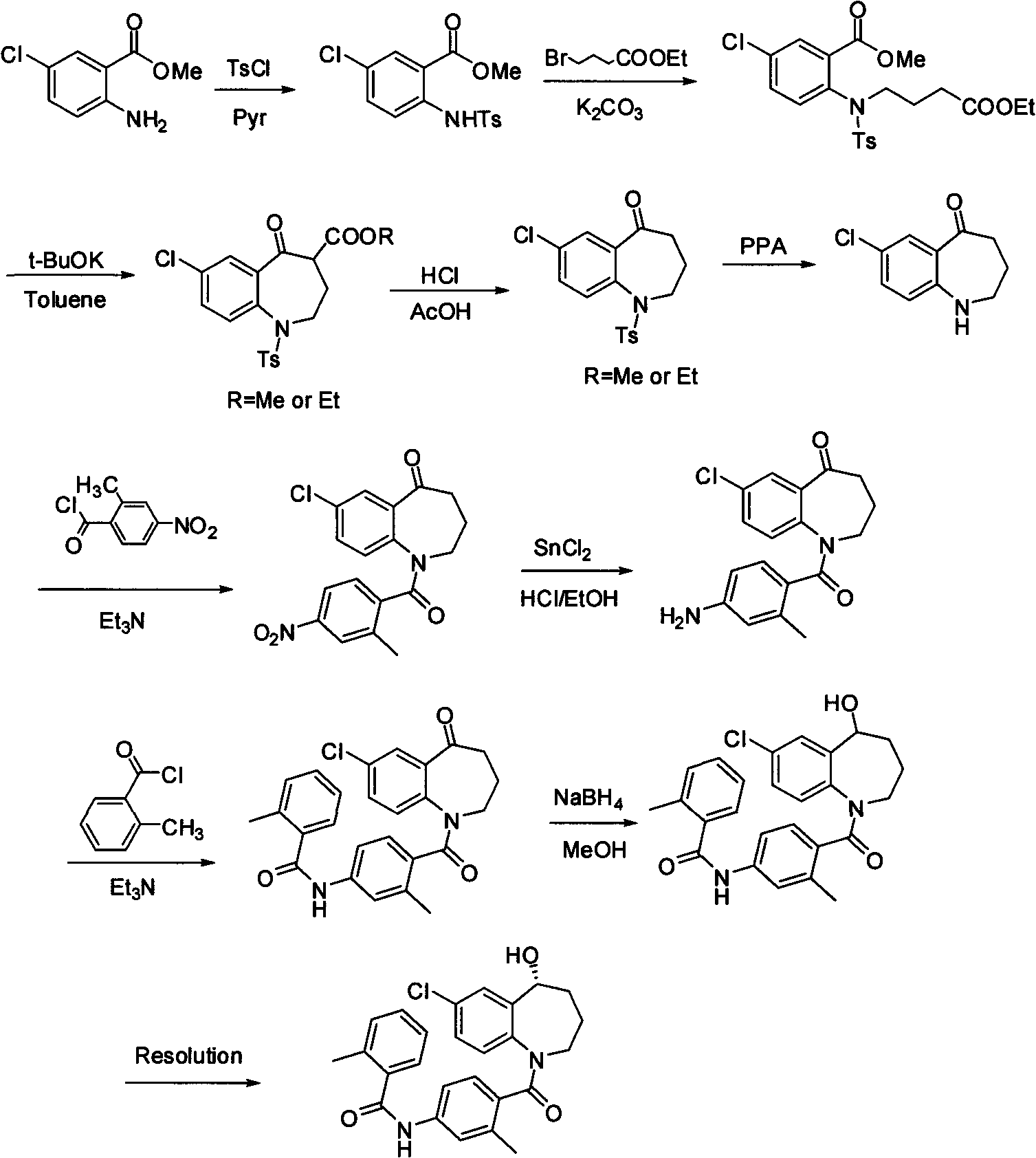
The invention relates to a preparation method of benzazepine compounds, particularly a preparation method of 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone. The 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone can be used as an intermediate for preparing a pitressin antagonist medicament Tolvaptan. The method comprises the following steps: by using parachloroaniline as a raw material, reacting with paratoluensulfonyl chloride in a condensation mode under the alkaline condition to firstly protect amino groups; under the alkaline condition, coupling with ethyl 4-bromobutyrate to obtain a compound; in the presence of alkali, hydrolyzing the obtained compound, and acidifying; in dichloromethane, preparing acyl chloride from the acidified compound under the action of thionyl chloride; under the action of Lewis acid, carrying out Friedel-Crafts acylation reaction to perform intramolecular cyclization; and finally, removing tosyl groups to obtain the target product. The invention has the advantages of simple technique and low cost, and is convenient for industrial large-scale production.
Owner:宁波人健药业集团股份有限公司
Treatment of wounds
InactiveUS7144721B1Avoid the needHydrolasesPeptide/protein ingredientsSerine Proteinase InhibitorsGly-Pro-Arg
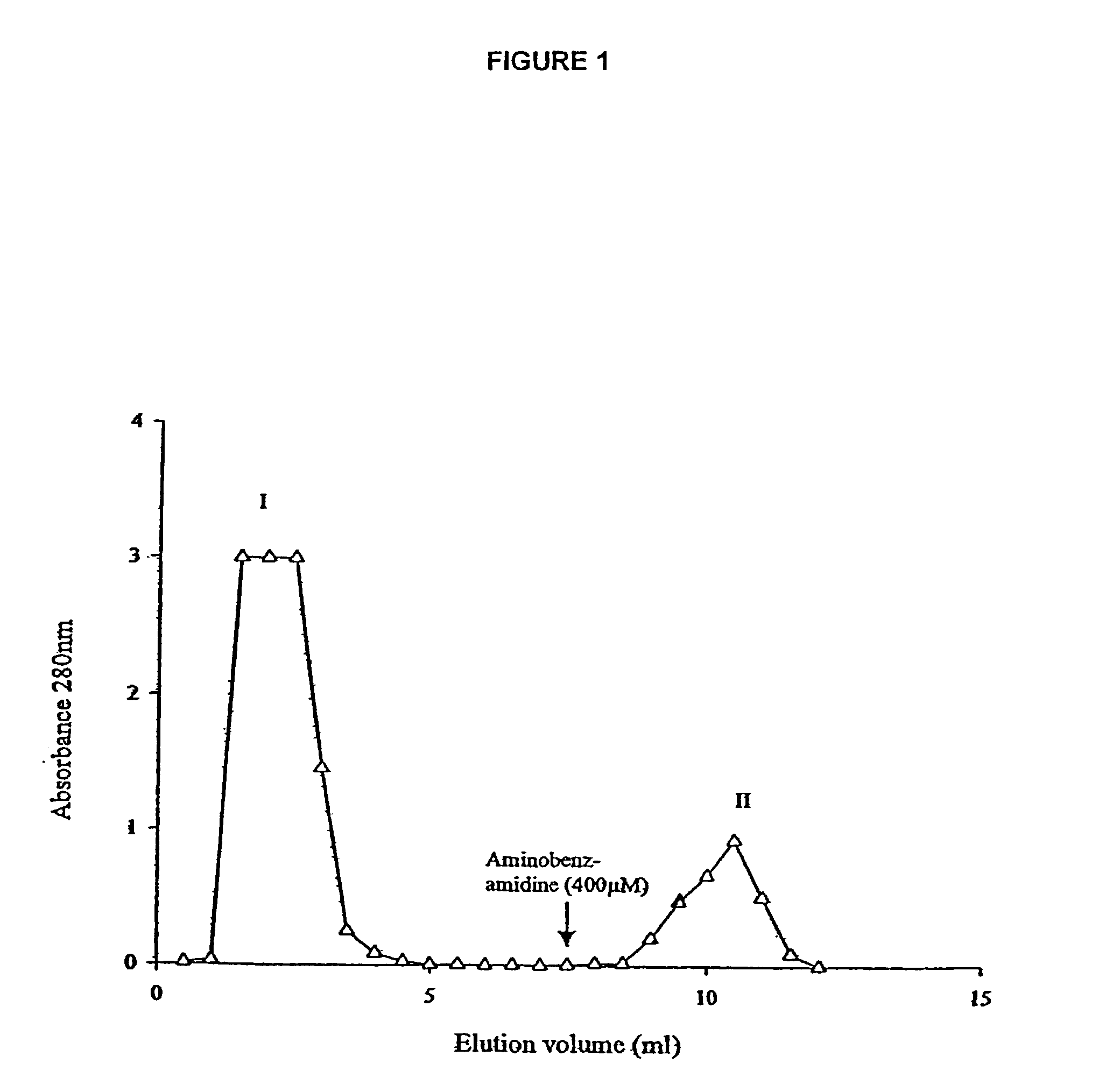
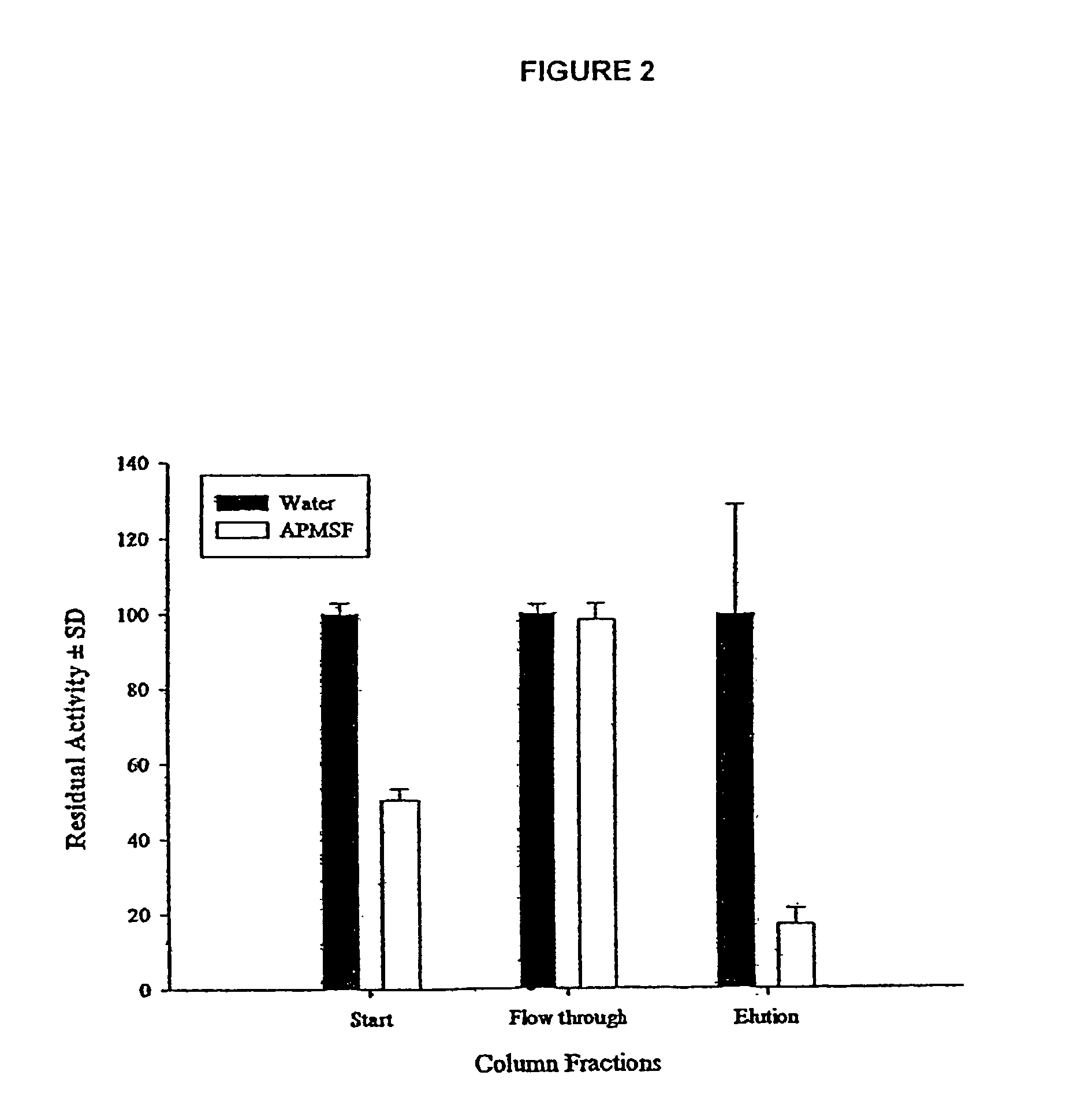
An isolated protein, for use in treatment of wounds, is characterized in that it is secreted by the organism Lucilia sericata and it exhibits proteolytic activity against FITC-casein at a pH of 8.0 to 8.5. The protein exhibits proteolytic activity against Tosyl-Gly-Pro-Arg-AMC but not against Suc-Ala-Ala-Phe-AMC, and its proteolytic activity against FITC-casein and Tosyl-Gly-Pro-Arg-AMC is inhibited by the serine proteinase inhibitors PMSF and AMPSF. The protein is also bound by immobilized aminobenzamidine.
Owner:DEFENCE THE SEC OF THE STATE OF +1
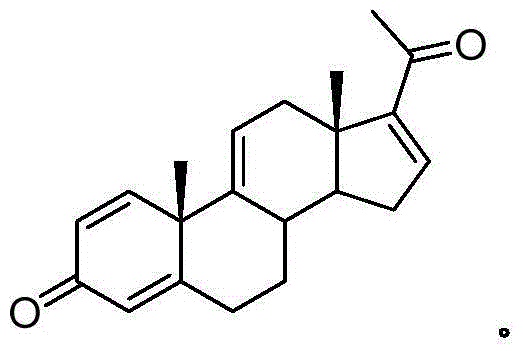
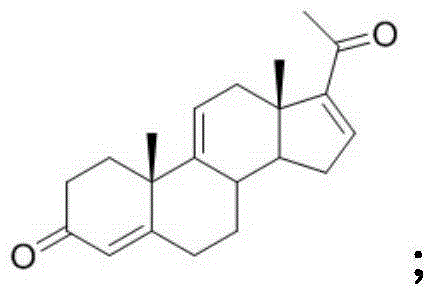
Synthetic method of ulipristal acetate
The invention relates to a synthetic method of ulipristal acetate. According to the method, 3, 3-(ethylenedioxy group) steroidal estrogen-5(10), 9(11)-diene17-ketone is used as a raw material and reacted with tosylmethyl isocyanide under the condition of alkalinity to obtain 3, 3-(ethylenedioxy group) steroidal estrogen-5(10), 9(11)-diene17-cyanogen; the 3, 3-(ethylenedioxy group) steroidal estrogen-5(10), 9(11)-diene17-cyanogen and a methyl grignard reagent are reacted to obtain methyl ketone; the methyl ketone and trialkyl ester phosphate are reacted in the oxidation environment under alkalinity conditions to obtain 3, 3-ethylenedioxy group-17 alpha-hydroxyl-19-norpregna-5(10), 9(11)-diene-20-ketone; the 3, 3-ethylenedioxy group-17 alpha-hydroxyl-19-norpregna-5(10), 9(11)-diene-20-ketone uses ethylene glycol to protect 20-carbonyl under the acid catalysis to obtain a key intermediate; and the intermediate is synthesized into a target product through four steps of reaction by means of known methods. The synthetic method is concise in paths, easy to operate, convenient to post-process, low in cost, high in total yield and easy to amplify, and raw materials are easily obtained.
Owner:SUZHOU KANGRUN PHARMA +1
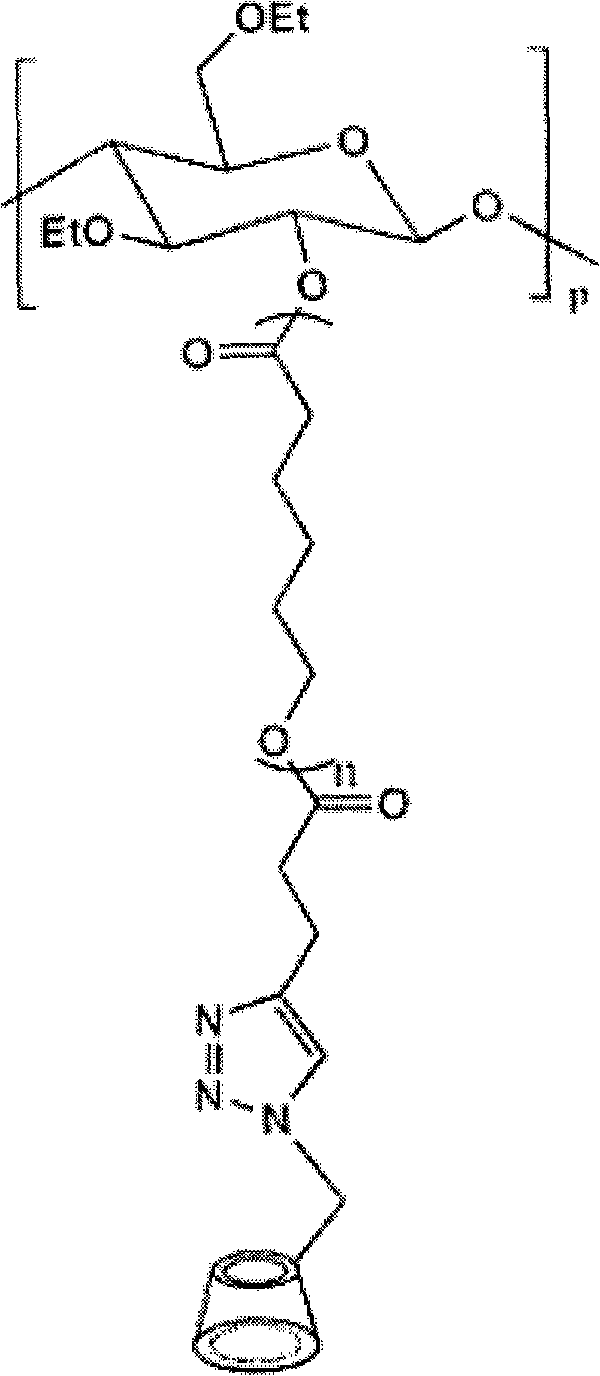
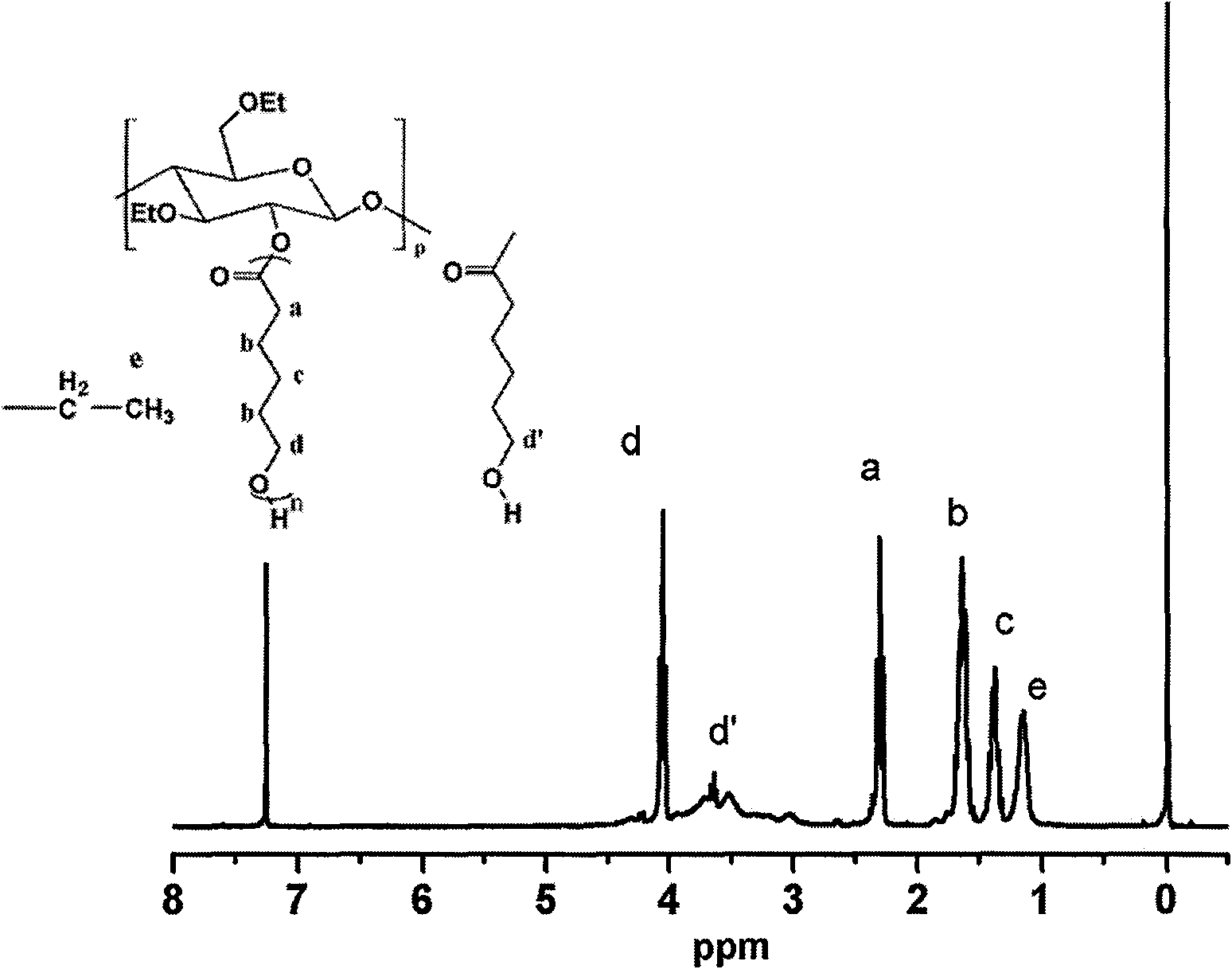
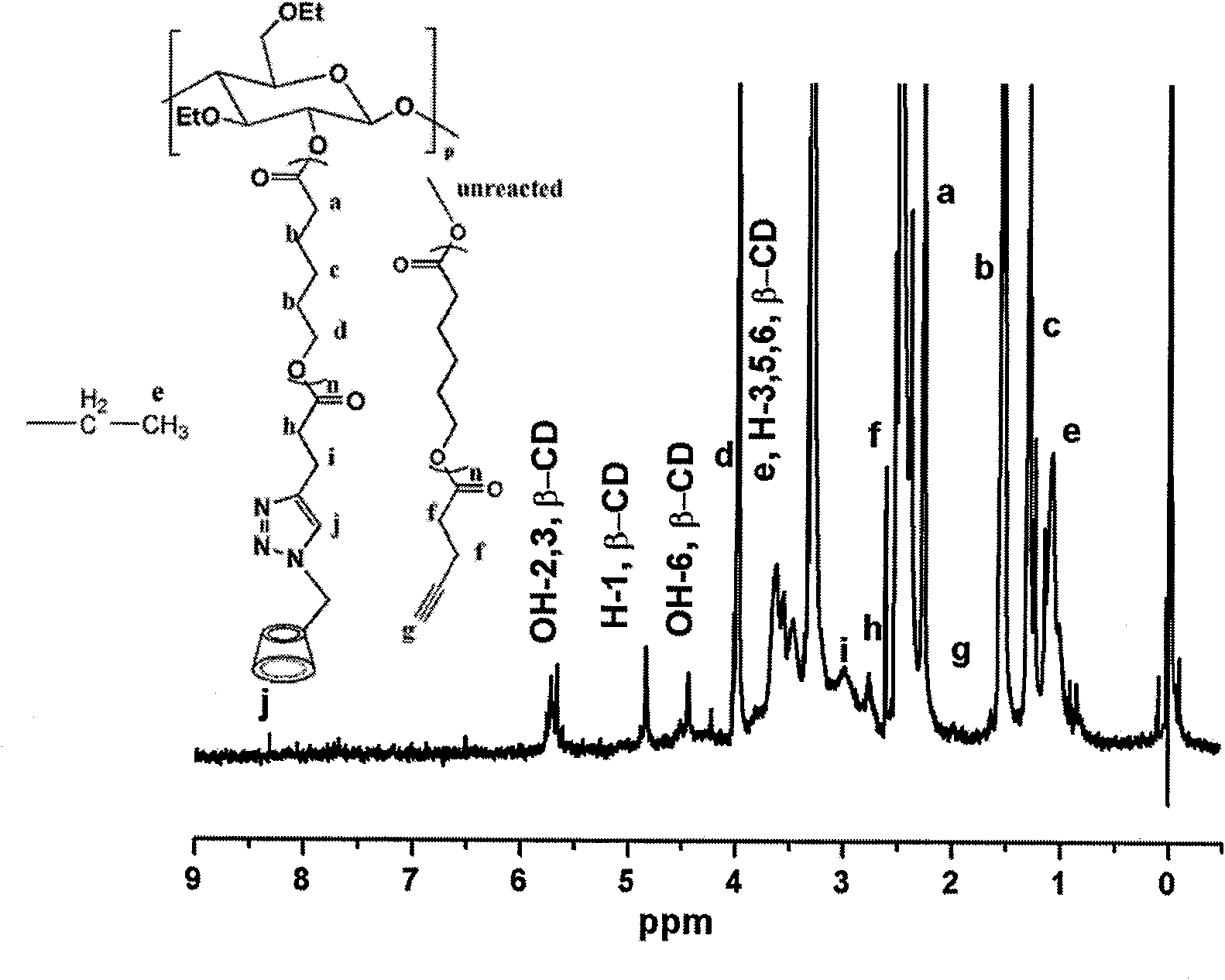
Cyclodextrin-bonded comb-shaped copolymer and preparation method thereof
The invention provides a cyclodextrin-bonded comb-shaped copolymer and a preparation method thereof, belonging to the field of macromolecule synthesis and assembly. The invention is characterized in that cyclodextrin-bonded comb-shaped copolymer is particularly beta-cyclodextrin-bonded ethyl cellulose-graft poly(epsilon-caprolactone) with the general formula thereof being shown in the following figure and the number-average molecular weight thereof being 30,000 to 500,000. The preparation method thereof comprises the following steps: firstly, synthetizing EC-g-PCL (ethyl cellulose-graft poly(epsilon-caprolactone)) by using the cationic ring-opening polymerization method; modifying the EC-g-PCL by an excessive amount of pentynoic acid; modifying the 6-hydroxy of beta-cyclodextrin by toluene sulfonyl chloride (TsCl); substituting sodium azide (NaN3) for the p-tosyl group on the cyclodextrin to obtain cyclodextrin azide; grafting the cyclodextrin azide onto a product B through the click chemical reaction, and dissolving the grafted product B in DMF (N,N-dimethylformamide), dialyzing and filtering to obtain the cyclodextrin-bonded comb-shaped copolymer. The cyclodextrin-bonded comb-shaped copolymer has the advantages of good biocompatibility, biodegradability, biostability and biofunctionality.
Owner:TSINGHUA UNIV
Method for performing ring-opening for cyclohexylaziridine by carboxylic acid
The invention discloses a method for performing ring-opening for cyclohexylaziridine by a carboxylic acid. The method includes that in a polar aprotic solvent system, alkali metal type inorganic base is utilized as a catalyst, a monocarboxylic acid is utilized as a nucleophilic reagent, and the cyclohexylaziridine which is activated by tosyl is subjected to a ring-opening reaction. The method has the advantages that the ring-opening reaction is simple in process, the reaction condition is mild, the solvent is environment-friendly, the carboxylic acid is utilized as the nucleophilic reagent, atom economical requirement of green chemistry is met, the catalytic agent is cheap, and the catalytic activity is high.
Owner:TAIYUAN UNIV OF TECH
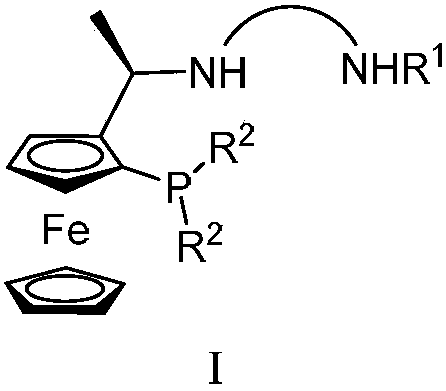
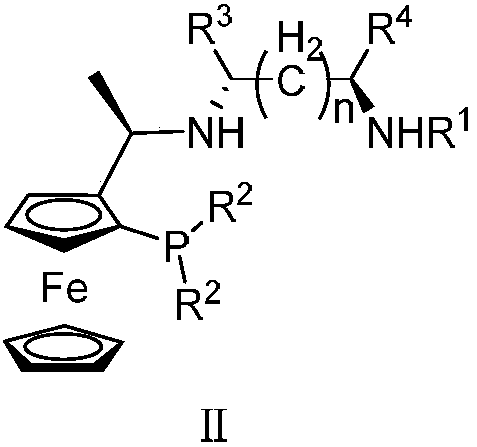
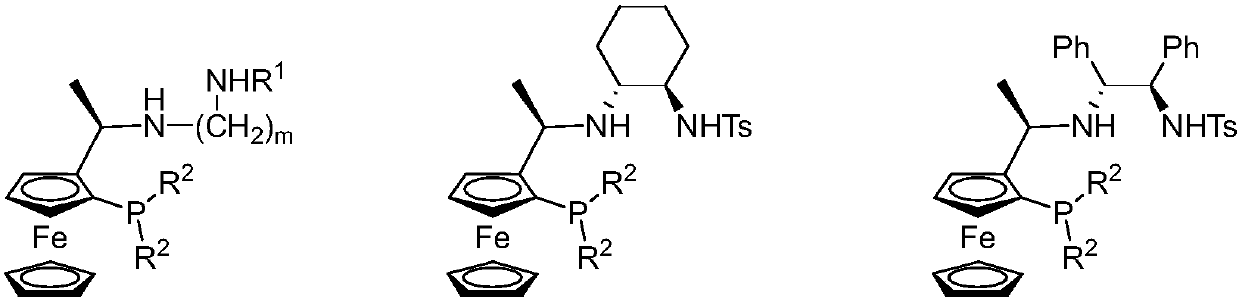
Tridentate nitrogen phosphine ligand and complex and application thereof in asymmetric catalytic hydrogenation of ketone
ActiveCN107722068AHigh enantioselectivityHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetoneOxygen
The invention belongs to the field of organic and medicine synthetic chemistry, and discloses a tridentate nitrogen phosphine ligand. The tridentate nitrogen phosphine ligand has a structure shown ina formula I which is shown in the attached figure, wherein R1 is toluene sulfonyl or 2,4,6-triisopropylbenzenesulfonyl, and R2 is aryl or substituted aryl. The invention also discloses a complex of the tridentate nitrogen phosphine ligand; the complex is prepared by mixing the tridentate nitrogen phosphine ligand and a transition metal complex; the complex is used for asymmetric catalytic hydrogenation of ketone. The tridentate nitrogen phosphine ligand has the advantages that 1, the synthesizing is easy, and the chiral ligand can be prepared by only two to three reaction steps; 2, the ligandis stable, the series of ligand is not sensitive to water and oxygen, and the convenience in storage and use is realized; 3, the catalyzing effect is good, and the catalyst can be used for realizing 100% of conversion and 99% of stereo selectivity on most of suitable primers; 4, the atom economy is high, and the activity of the catalysis system is higher; for most of suitable primers, the conversion number reaches more than 10000, and the maximum conversion number reaches 200000.
Owner:SHENZHEN CATALYS SCI & TECH CO LTD
Preparation method of Quizalofop-p-ethyl with high optical content
ActiveCN101531640AHigh optical purityQuick responseBiocideOrganic chemistryQuinoxalineOrganic solvent
The invention relates to a preparation method of Quizalofop-p-ethyl with high optical content. In the method, 6-chorine-(4-hydroxyl group phenoxyl) quinoxaline and S(-)p-toluenesulfonyl ethyl lactate are reacted in non-polar organic solvent for 4-8h under the temperature of 60-150 DEG C with metal carbonate of 100-800mesh as the catalyst. The optical antimer proportion of the Quizalofop-p-ethyl prepared by the method R:S is more than 98.5:1.5, which is suitable for industrial mass production, is easy for operation and low in cost.
Owner:NUTRICHEM LAB CO LTD
6-(2-glucosyl amido)-beta- cyclodextrin derivant and method for producing the same
The invention relates to a 6-(2-glucosyl amido)-beta-cyclodextrin derivative and a relative preparation method, belonging to organic functional material technical field. The preparation method comprises dissolving 6-O-tosyl-beta-cyclodextrin, glucosamine hydrochloride, triethylamine and potassium iodide in pyridine, arranging the mixture in a sealed reactor, using N2 to replace the air in the reactor until the oxygen content of the reactor is lower than 100ppm, raising temperature and reacting, depressurizing, distilling and recovering pyridine to obtain mixed product, dissolving the mixed product via water completely, adding the solution in an acetone-methanol mixture, reacting and depositing to obtain crude product, electrically dialyzing at standard electricity, atomizing and drying to obtain the final product. The inventive cyclodextrin derivative has better solubility and stability, which can be widely used for drug, food, cosmetic product, spice, daily necessaries and material fields.
Owner:SHANDONG UNIV
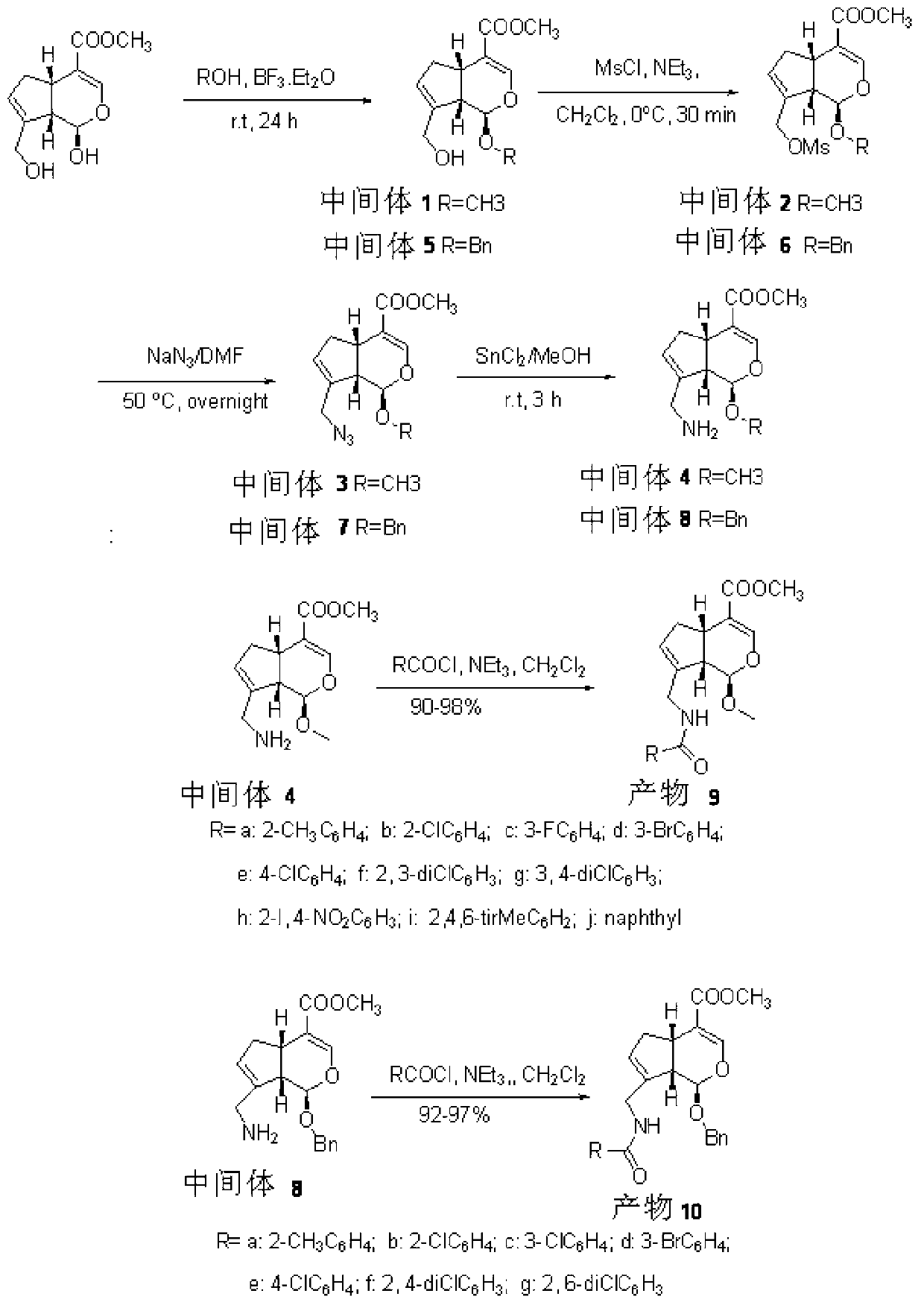
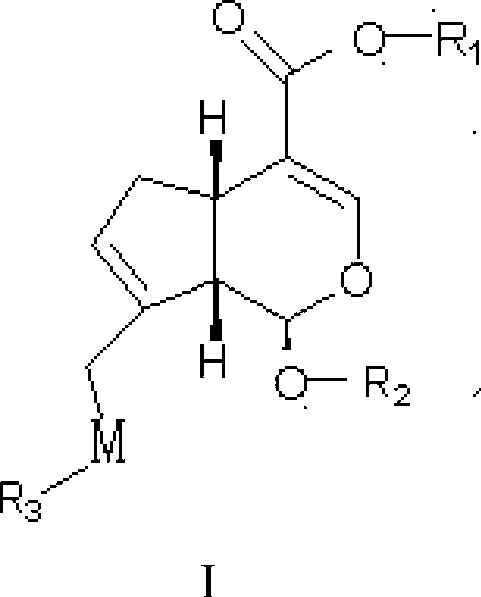
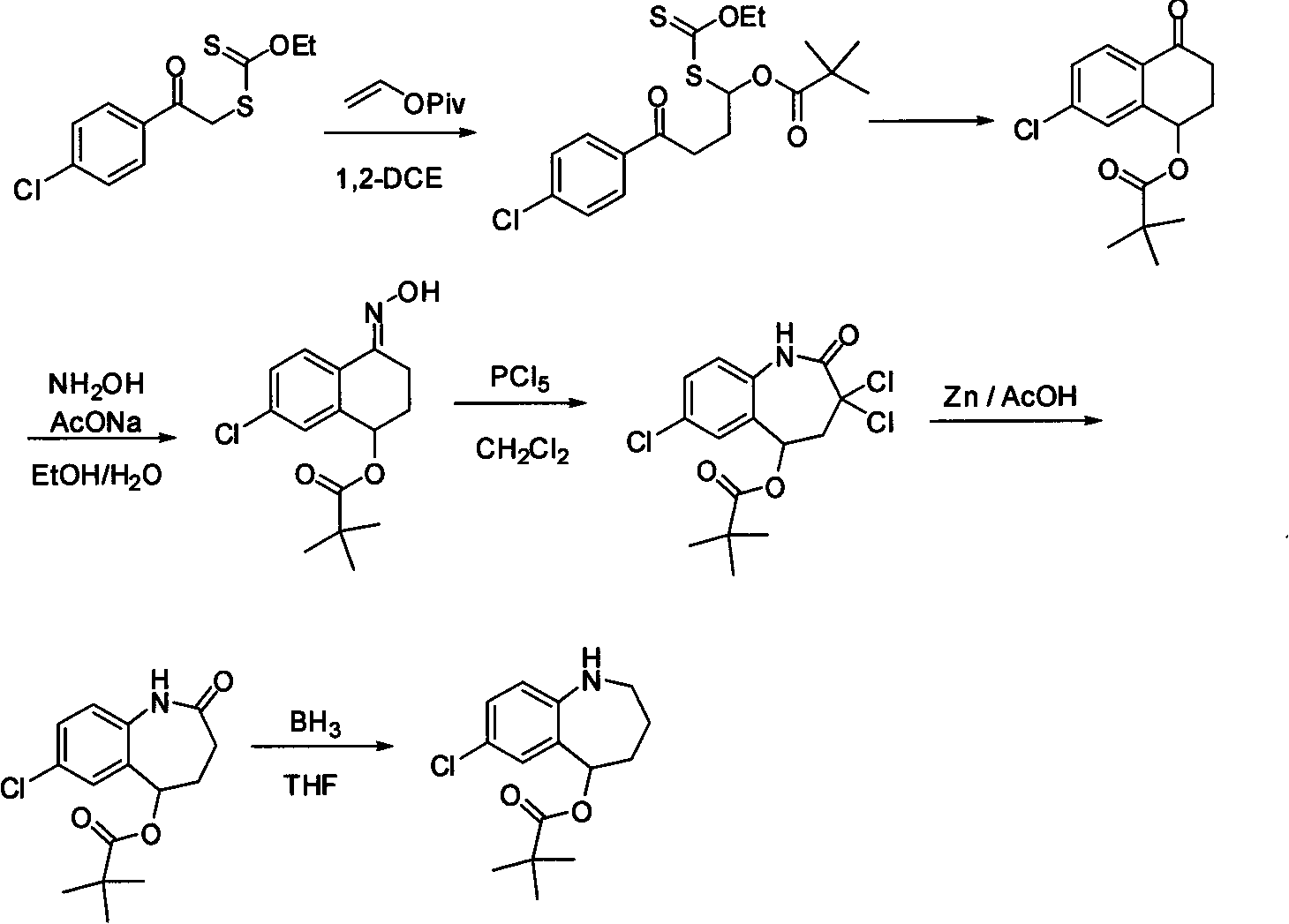
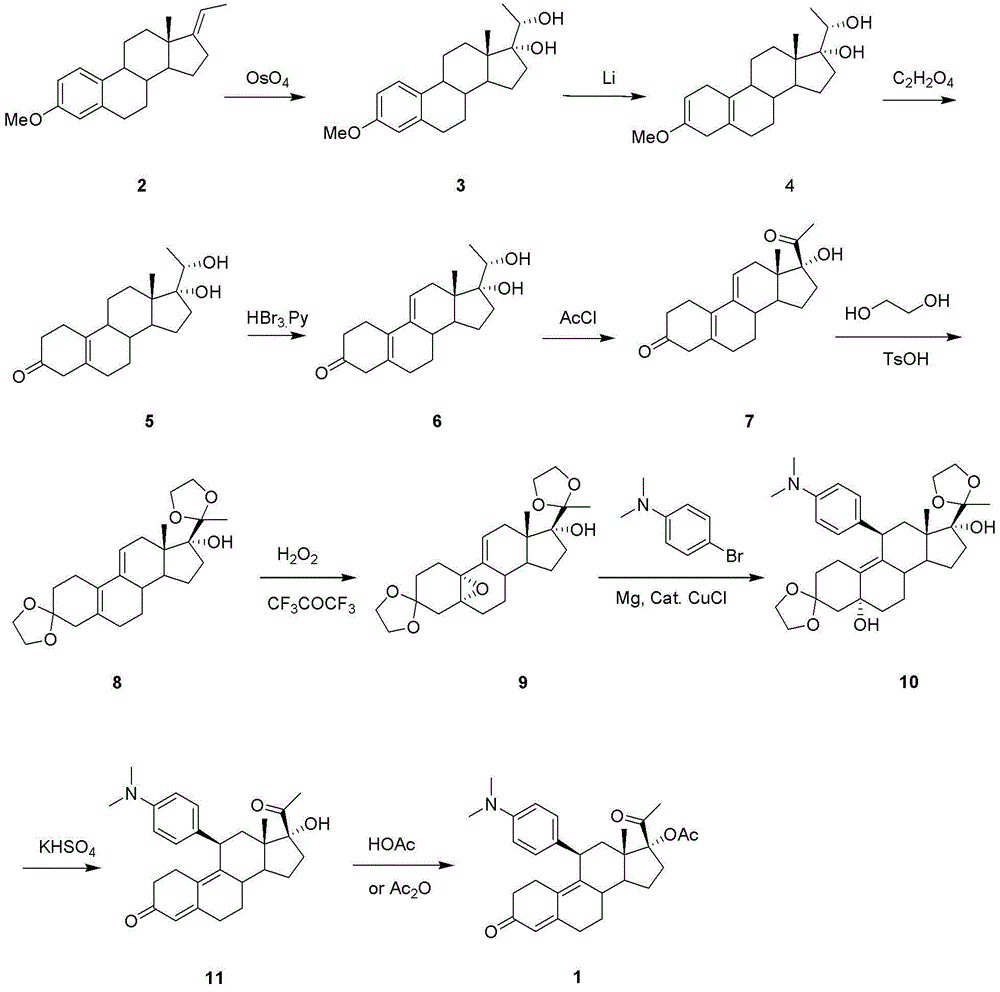
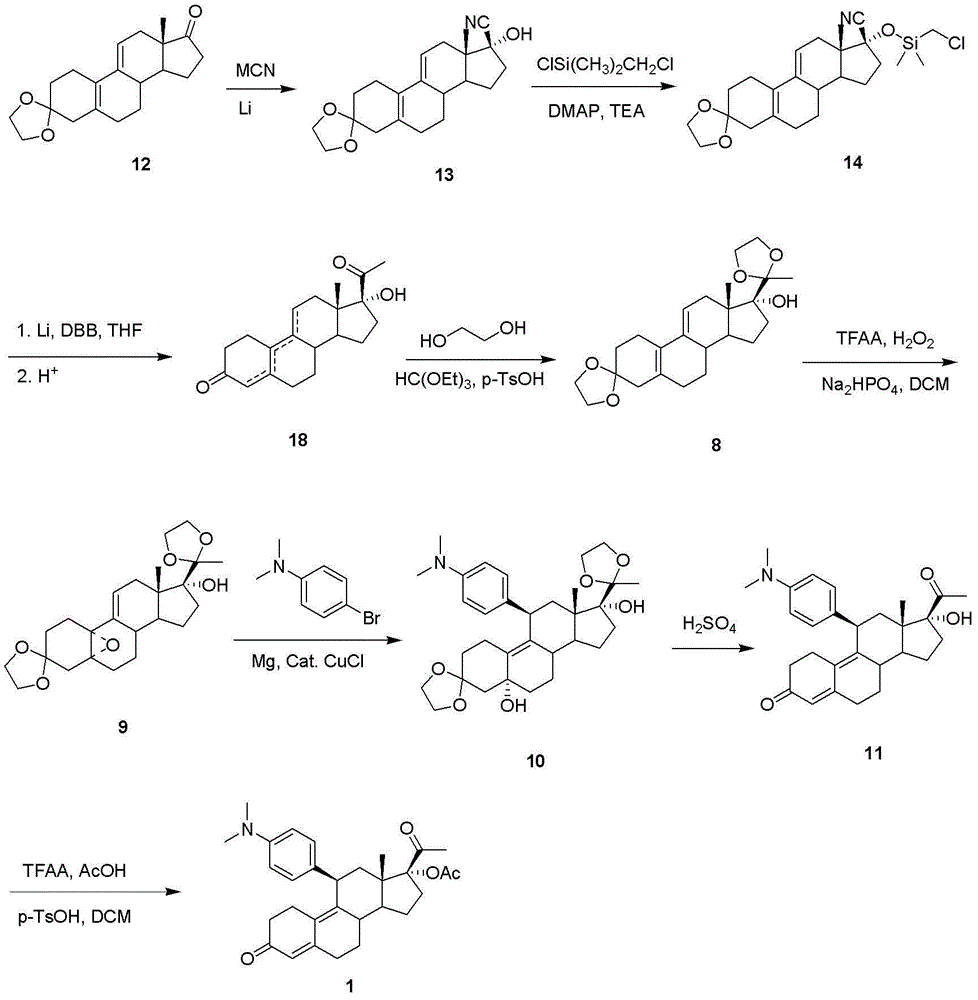
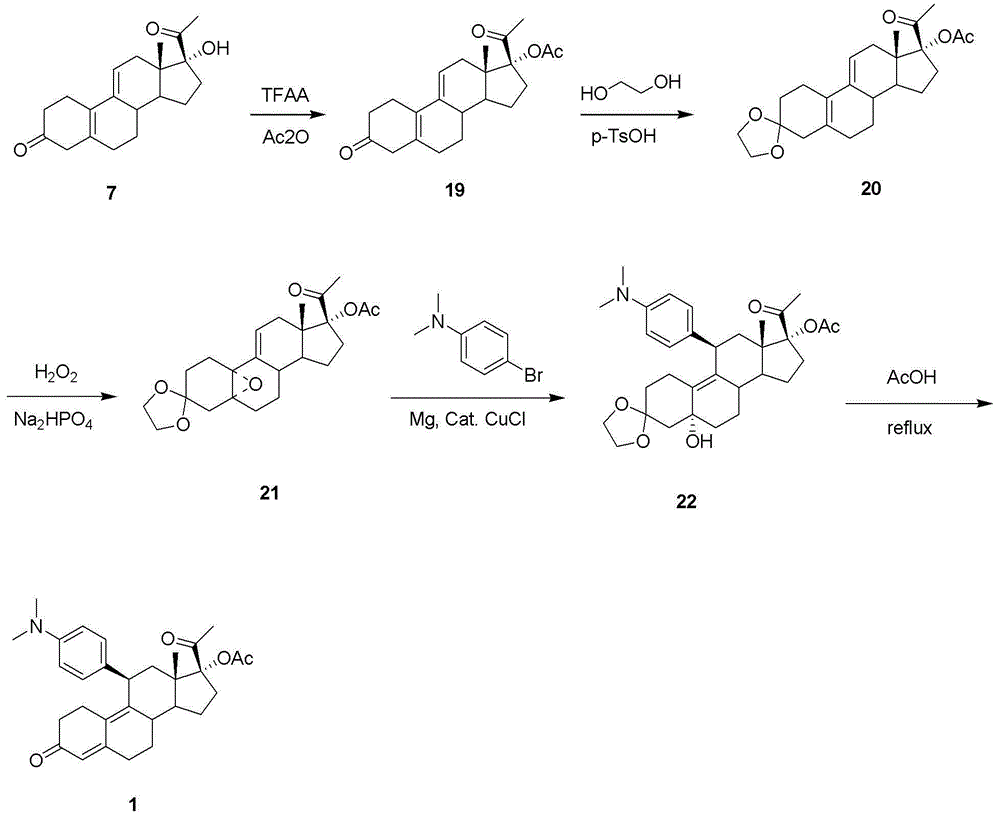
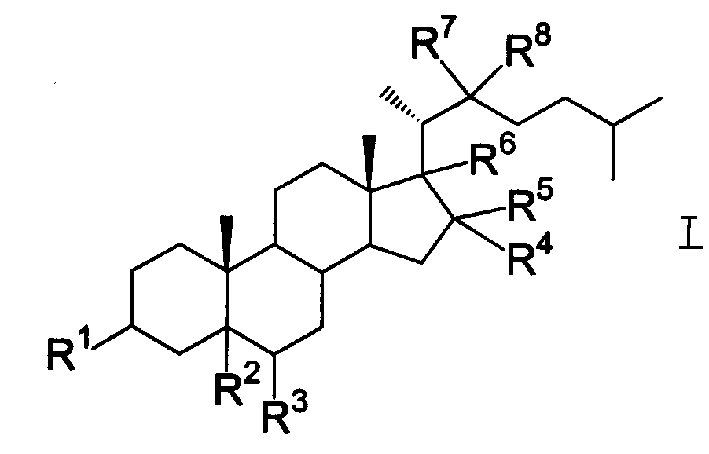
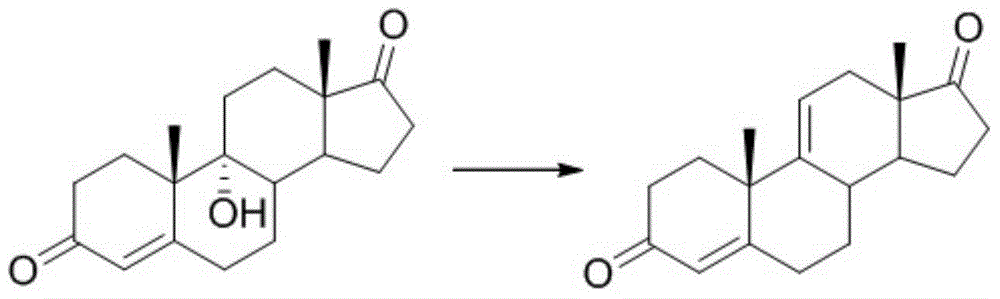
Steride, its systhesis method and use
A kind of compound, synthesizing method and its use is disclosed in the invention, whose structure formula is 1:R1=OH, OAc, OMs, OTs, OTBS, OTBDPS etc. or R1, R2 forming 3,5-three-membered ring structure; R2=x, or R1, R2 forming 1,5-three-membered ring structure, or R2, R3 forming 5,6-double bonds; R3=OH, OAc, OMs, OTs, OTBS, OTBDPS X etc. or R2, R3 forming 5,6-double bonds; R4=H, OH, SPh, S(CH2)nSA etc. or R4, R5 forming carbonic bonds, or R4, R5=S(CH2)nS, (n=2,3, etc.), or R5, R6 forming 16, 17 double bonds, or R5, R7 forming 16, 22 aether bond; R6=H, or R5, R6 forming 16, 17 double bonds; R5, R7 forming 16, 22 aether bond, or R7, R8 forming carbonic bonds; R5=H or R7, R8 forming carbonic bonds. Thereinto, Ac is acetyl, Ms is methylsulfonyl, Ts is toluenesulfonyl, TBS is butyl dimethylsilyl, TBDSPS is butyl dipheny Si-based, X is halogen, SPh is phenylthio.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
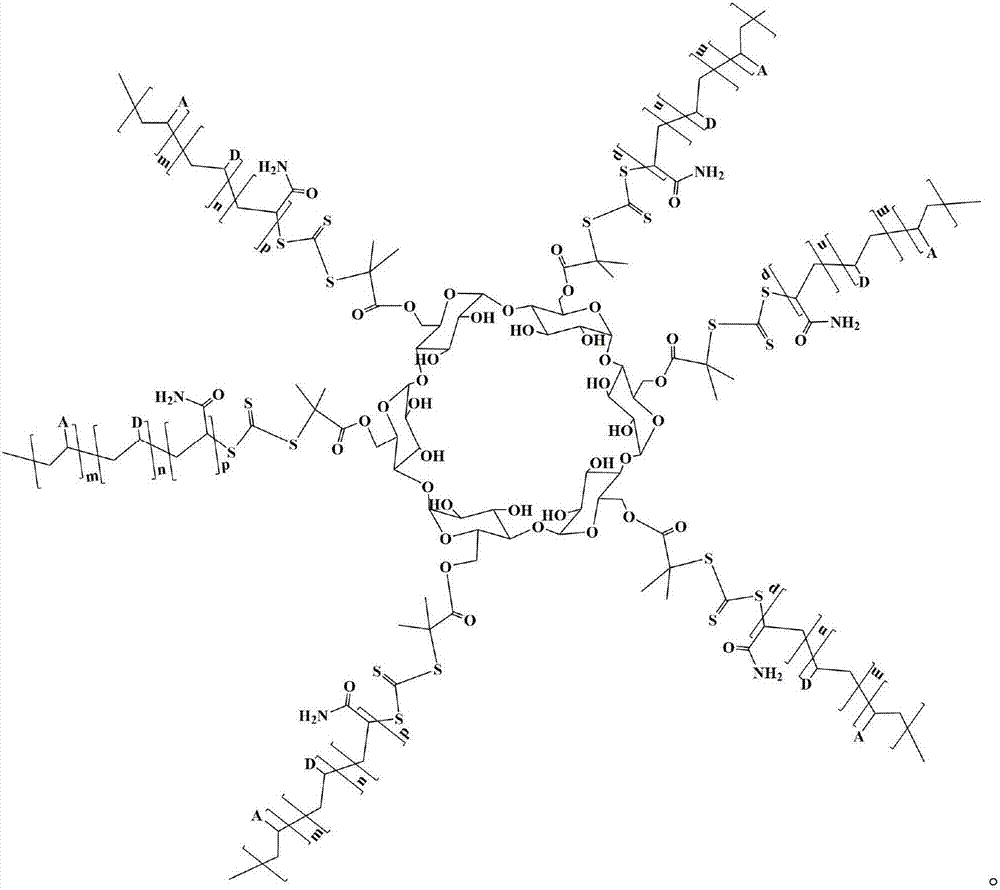
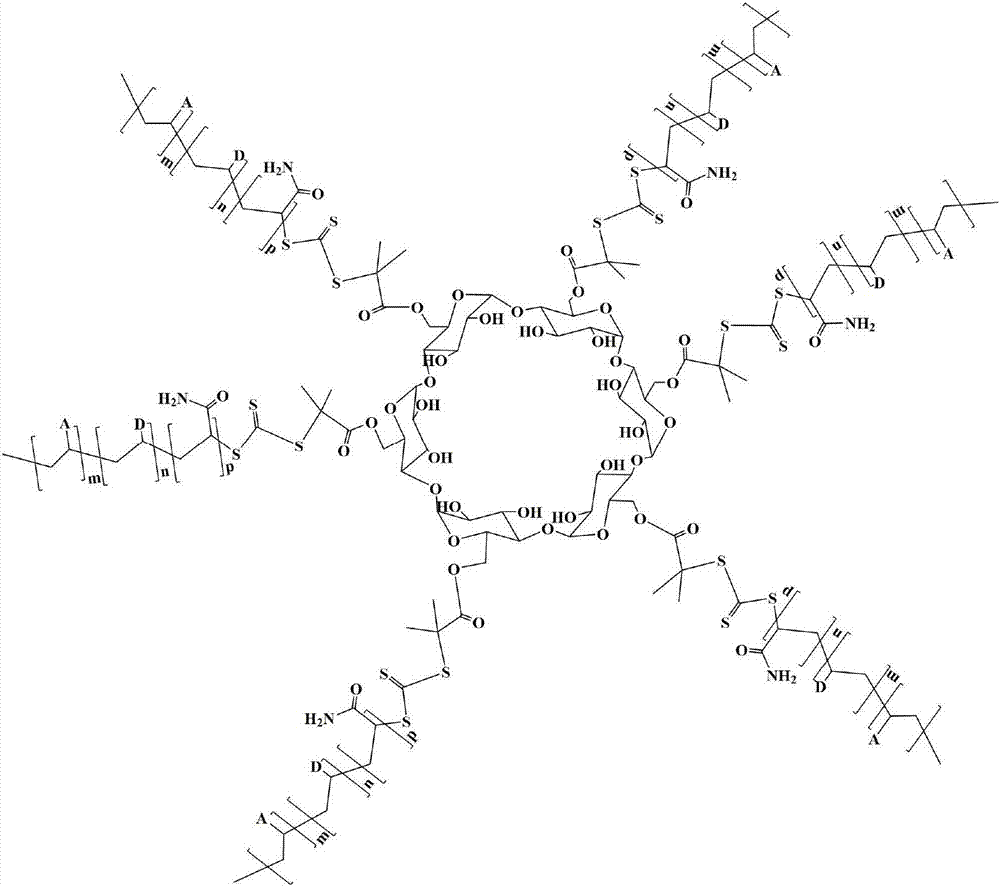
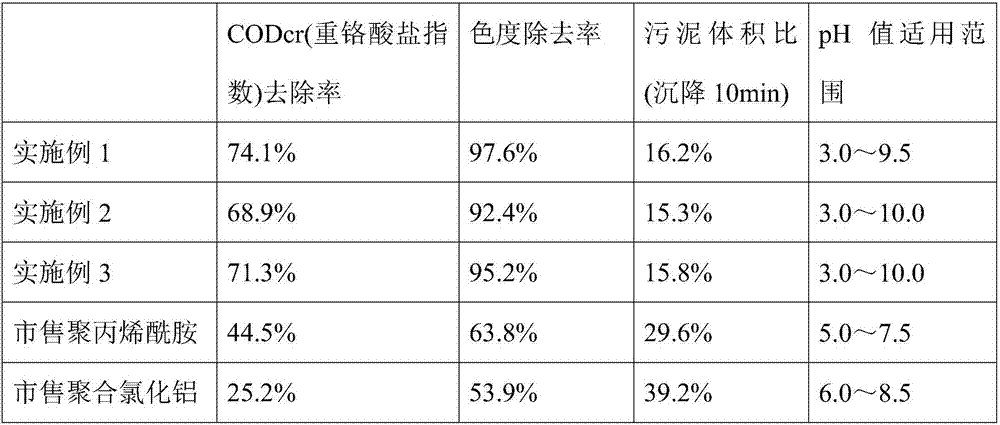
Star type copolymer sewage treatment agent using cyclodextrin as core and RAFT preparation method of star type copolymer sewage treatment agent
InactiveCN107487830AImprove performanceGood flocculation effectWater/sewage treatment by flocculation/precipitationCyclodextrinTosyl
The invention relates to a star type copolymer sewage treatment agent using cyclodextrin as a core and an RAFT preparation method of the star type copolymer sewage treatment agent. In the invention, cyclodextrin is used as the core, each glucose unit of the cyclodextrin is connected with an acrylamide copolymer molecular chain, the average molecular weight is 300,000-800,000, and the polydispersity coefficient is less than 1.5. The preparation method comprises the following steps: firstly, reacting acyl chloride-containing dithioester or trithioester with diamine to prepare amino-substituted dithioester or trithioester, and then reacting amino-substituted dithioester or trithioester with 6-(p-toluenesulfonyl) cyclodextrin to prepare a star type RAFT reagent; and then initiating aqueous solution polymerization of a monomer to prepare a star type copolymer under the action of an initiator and the star type RAFT reagent. The copolymer sewage treatment agent has the advantages of stable performance and high flocculation efficiency.
Owner:内蒙古信尚环保科技有限公司
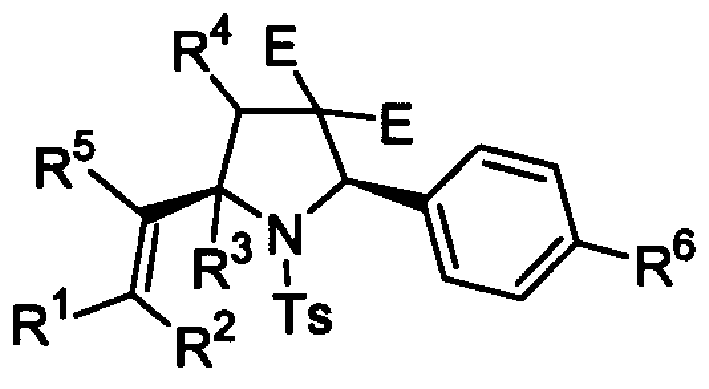
2,5-cis-bisubstituted pyrrolidine derivative and its preparing process and usage
The present invention relates to a 2.5-cis-substituted pyrrolidine derivative, its synthesizing process "Multi-component one reactor method" and its application in synthesizing molecular blocks of chiral adjuvant or ligand, or the compound containing bioactive pyrrolidinecycle.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
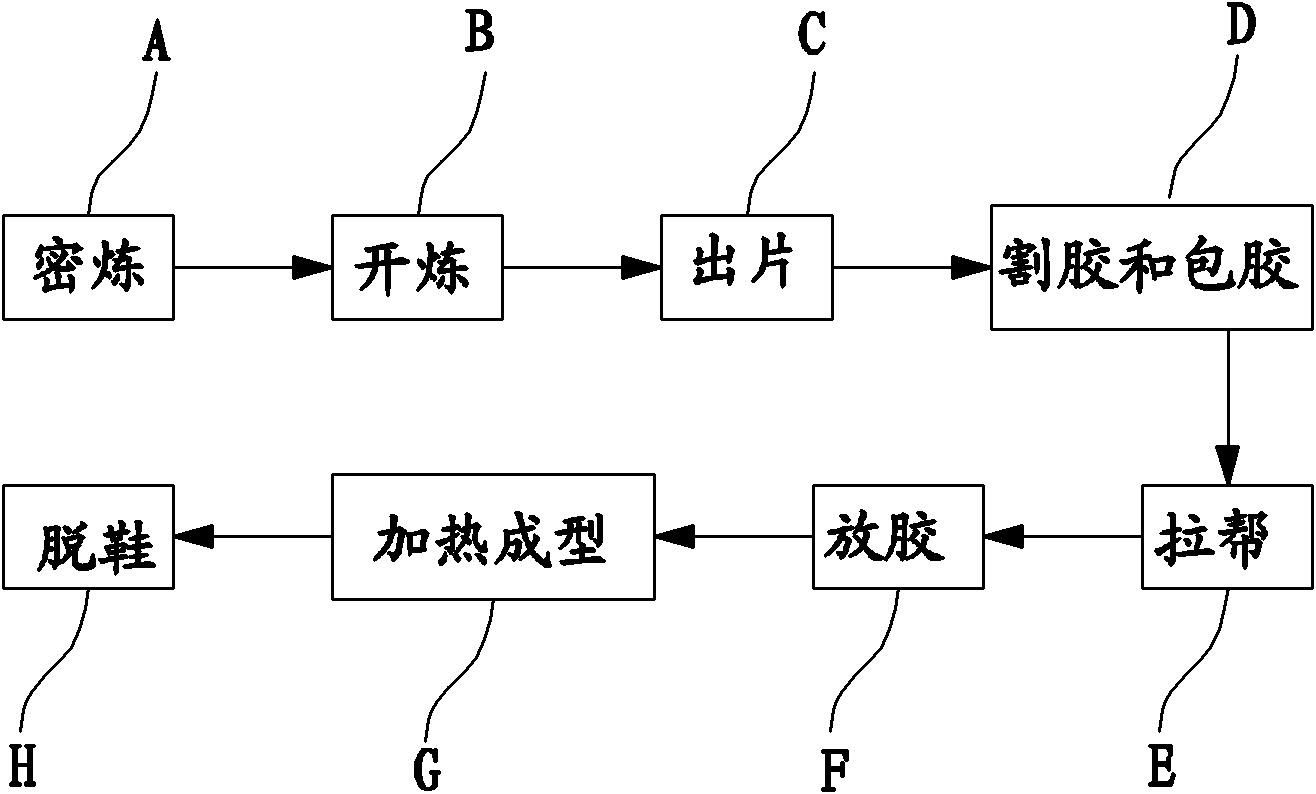
Sole formula and production process thereof
The invention discloses a sole formula and a production process thereof. The formula comprises the following components: 3-5 parts of natural rubber, 10-13.8 parts of isoprene rubber, 5-7 parts of talc powder, 10-14.5 parts of nano ointment, 3-5.6 parts of white carbon black, 0.94-1.2 parts of zinc carbonate, 0.1-0.3 part of flow promoter, 0.01-0.03 part of DCP (Dicumyl Peroxide), 0.188-0.276 part of anti-aging agent, 0.125-0.25 part of protective wax, 0.05-0.08 part of p-toluenesulfonyl, 0.1-0.2 part of sulfur and 0.025-0.05 part of foaming agent. The production process comprises the procedures of banburying, open milling, sheet discharging, rubber tapping, rubber coating, bringing up, sizing, heating, molding, taking off shoes and the like. The sole produced by adopting the production process has the characteristics of high binding property, high elasticity, high stretching strength, high tear resistance, high electrical insulating property, high wear resistance, high drought resistance, low temperature resistance, noise resistance, high processability and the like.
Owner:福建晋江市越峰鞋塑有限公司
Polycation gene carrier as well as preparation method and application thereof
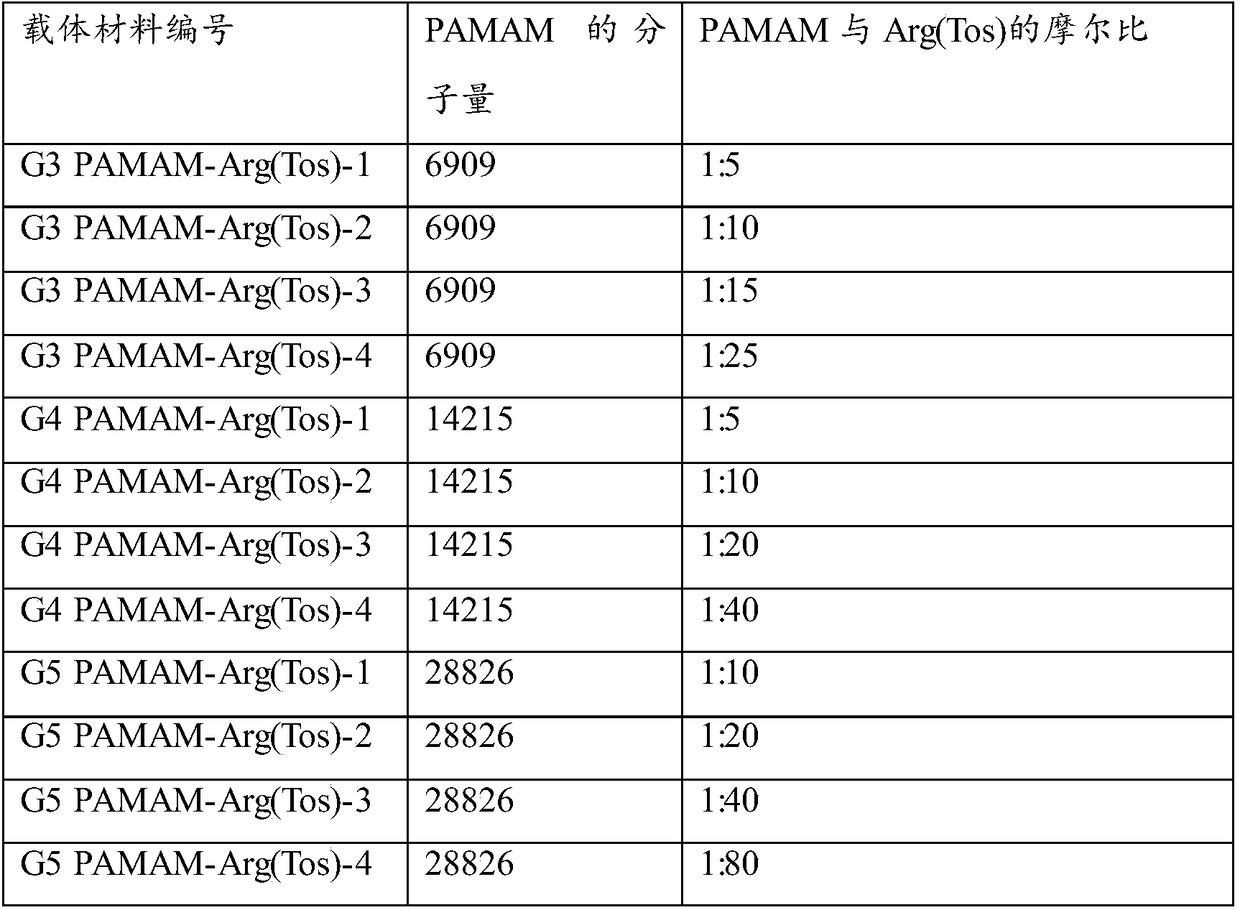
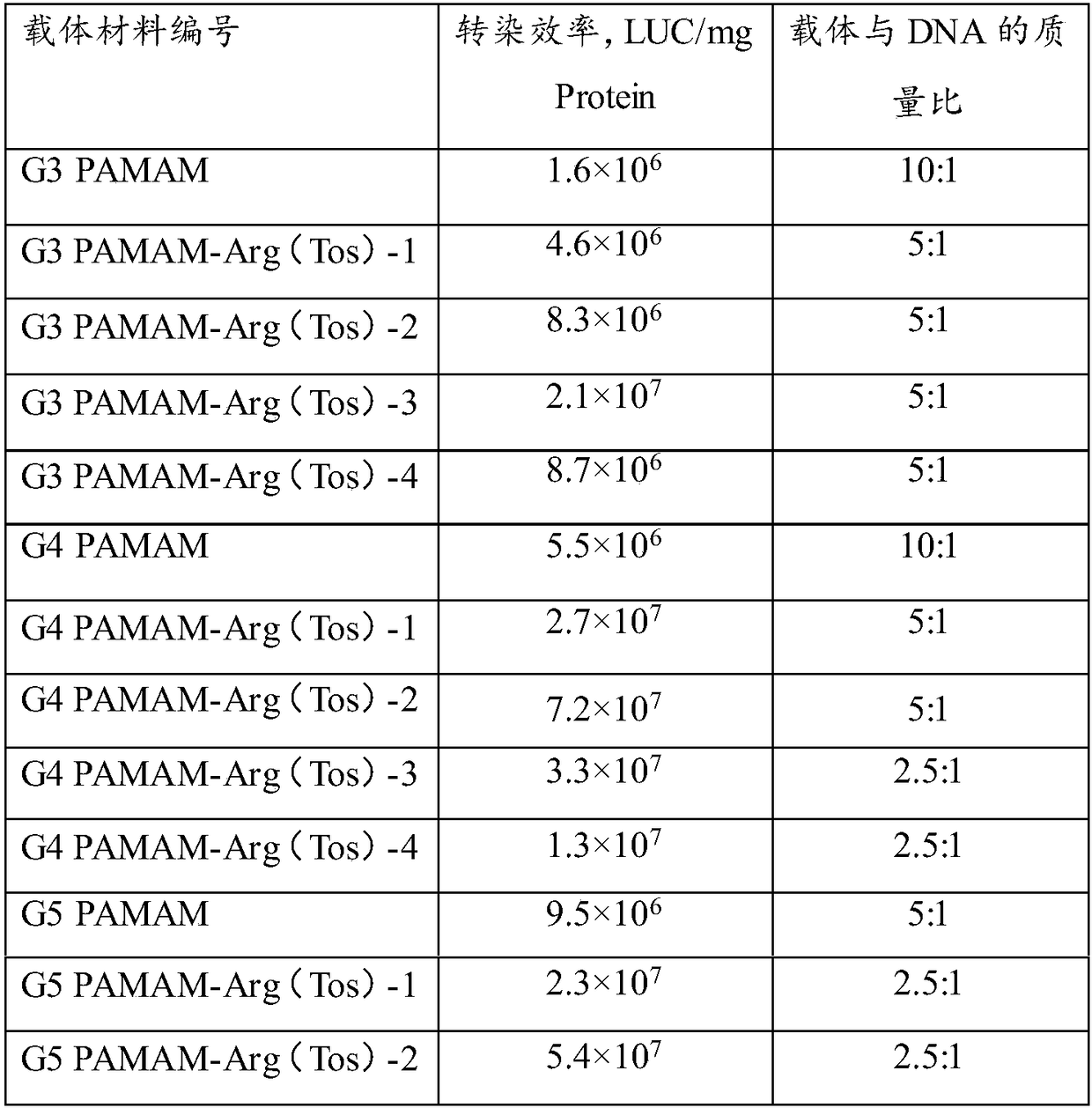
ActiveCN108728496AEasy to makeGood repeatabilityGenetic material ingredientsOther foreign material introduction processesArgininePolyamide
The invention provides a polycation gene carrier as well as a preparation method and application thereof. The polycation gene carrier comprises a grafting framework and arginine which is grafted withthe grafting framework and is used for protecting tosyl; raw materials of the grafted raw materials are selected from branch type polyamide-amine, hyperbranched polylysine with the molecular weight being 2000 to 100000 g / mol or linear epsilon-polylysine with the molecular weight being 2000 to 50000 g / mol; the branch type polyamide-amine is selected from one or several kinds of materials of types of PAMAM-G3, PAMAM-G4 and PAMAM-G5. The polycation gene carrier provided by the invention is grafted with the arginine for protecting tosyl onto the specific grafting framework; the transfection efficiency of the compound nanometer particles prepared from the polycation carriers can be obviously improved. In addition, the charge density of the nanometer compound particles can be effectively reduced; the cell toxicity can be further reduced.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Synthesis methods of lorcaserin derivative and salt thereof
The invention discloses a synthesis method of a lorcaserin derivative. The synthesis method comprises the following steps: resolving a compound shown as a formula (II) under the action of an optical resolving agent; performing post-treatment to obtain the lorcaserin derivative. In the formula (II), R is H, alkyl with 1-4 carbon atoms, halogen, methoxyl or nitryl; R2 is H, alkyl with 1-4 carbon atoms, methoxyl, carboxybenzyl, t-butyloxycarboryl, methylsulfonyl, tosyl and substituted or unsubstituted benzyl; the optical resolving agent is acyl-substituted tartaric acid. The revolving efficiency is increased by selecting the optical resolving agent having a specific structure, and an optical pure product of which the ee value is over 99 percent can be obtained by means of simple recrystallization; meanwhile, resolving operation is easy, a single solvent is used for resolving, materials are fed into one pot, and the method is suitable for industrial production. The invention further discloses a synthesis method of a salt of the lorcaserin derivative. The obtained salt of the lorcaserin derivative can be applied to preparation of novel weight-reducing medicaments.
Owner:CHINA JILIANG UNIV +1
N-(4-toluenesulfonyl)-1,2-diphenyl ethylenediamine functionalized hollow PMO (Periodic Mesoporous Organosilica) catalyst preparation method
InactiveCN103433074AGood dispersionNo pollutionCatalyst carriersOrganic-compounds/hydrides/coordination-complexes catalystsReaction speedReaction system
The invention discloses a preparation method of an N-(4-toluenesulfonyl)-1,2-diphenyl ethylenediamine functionalized hollow PMO (Periodic Mesoporous Organosilica) catalyst. The hollow PMO catalyst can be better dispersed into an organic or inorganic solvent, as a result, compared with a traditional PMO material, the hollow PMO catalyst can remarkably increase the reaction speed on the basis of the speed of the traditional PMO catalyst. The catalyst prepared by the preparation method has the following advantages: (1) the reaction speed is further increased; (2) a series of catalysts can be synthesized through coordinating different metals with the catalyst; (3) the catalyst can be better dispersed into a reaction system.
Owner:SHANGHAI NORMAL UNIVERSITY
Radioactive molecular probe taking TSPO as target point as well as preparation method and application thereof
InactiveCN111116595AControl permeabilityImprove dynamic propertiesOrganic chemistry methodsRadioactive preparation carriersSide chainDisease patient
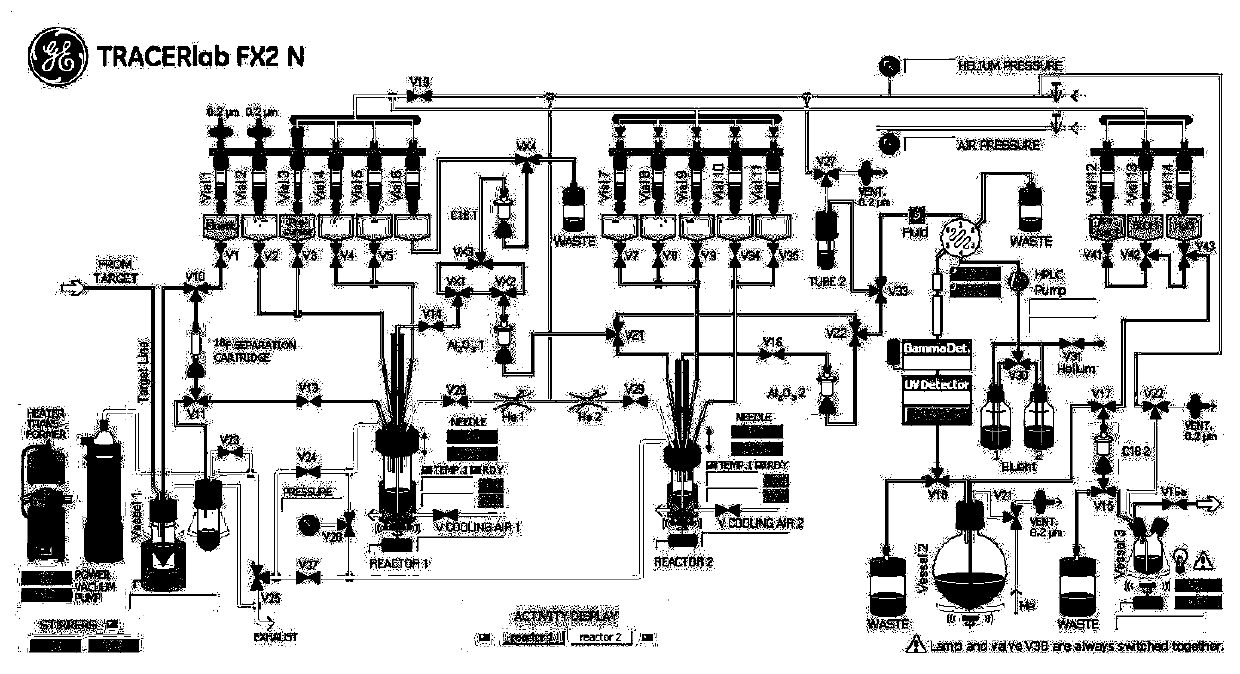
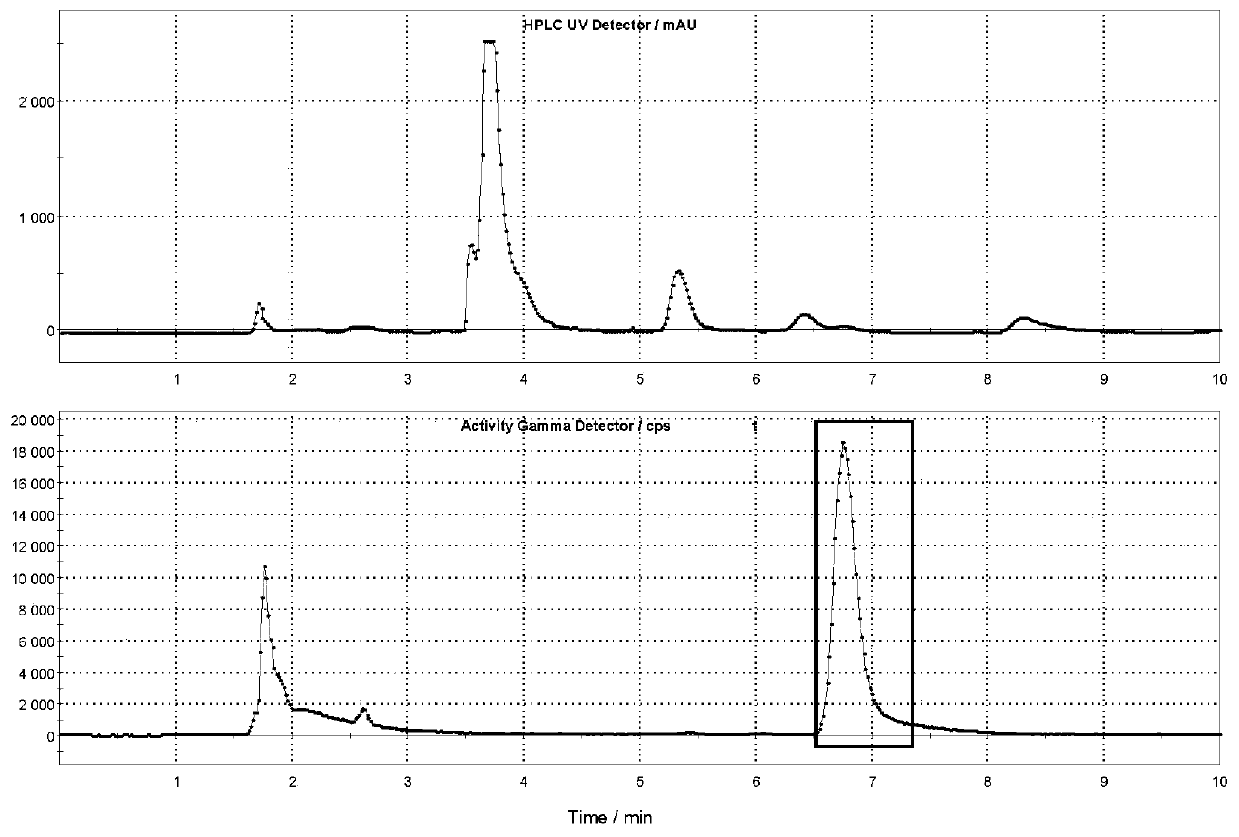
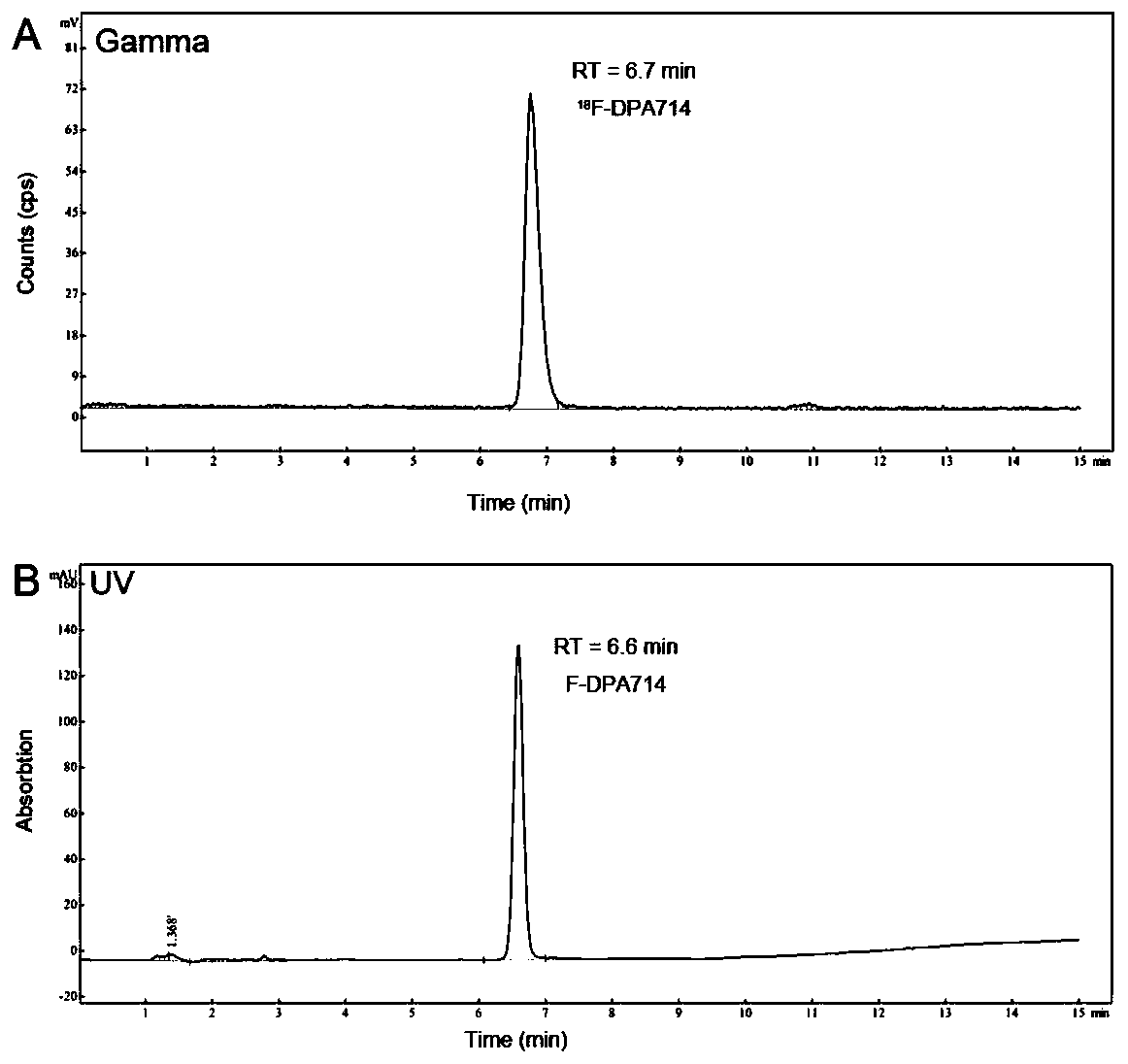
The invention discloses a small molecular organic compound with a specific targeting TSPO (Transfer Protein). The structure of the small molecular organic compound comprises a benzene ring, pyrazolo imidazole and a diethyl acetamide structure. The P-toluenesulfonyl on a side chain of a benzene ring is used as a marker site; carrier-free radioactive fluorine-18 is used as a radioactive source; andmild alkaline reaction conditions are designed and utilized. By using a functional GE TRACERlab FX2N synthesizer, a target compound [18F] DPA714 is obtained through a one-step reaction. The method hasthe advantages of mild reaction conditions, simple steps, and automatic labeling; the non-attenuation correction yield reaches 40% or above; the radiochemical purity of the product is higher than 99%, the specific activity is higher than 148GBq / [mu] mol (4Ci / [mu] mol). The invention solves the technical problems of low labeling yield, low specific activity, complex operation and the like in the prior art. A radioactivity molecular probe prepared from the small molecular compound is shown as an imaging evaluation result of a positron emission tomography (PET) in patients (including Alzheimer'sdisease and cerebral apoplexy) with neuroinflammation related diseases, and the radioactivity molecular probe has the characteristics of high sensitivity, high sensitivity, high sensitivity, high sensitivity, high sensitivity, high sensitivity and the like. It is proved that the molecular probe has good specificity, targeting, stability and non-target tissue clearance rate.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
Synthesis method and intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone
ActiveCN104628808AReduce usageRaw materials are cheap and easy to getSteroids preparationBulk chemical productionSynthesis methodsSide chain
The invention relates to a synthesis method and main intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. The synthesis method sequentially comprises the following steps of reacting a second intermediate and tosylmethyl isocyanide in an organic solvent at the temperature of lower than 35 DEG C below zero to generate a third intermediate; reacting the third intermediate and a methylated reagent in an organic solvent at the temperature of 70-90 DEG C, and then, removing methyl ether protecting groups and tosylmethyl isocyanide under the action of an acid to obtain a fourth intermediate; and reacting the fourth intermreidate under the action of 3-ketosteroid-1-dehydrogenase to generate pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. Raw materials of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone are cheap and available; the yield of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone is relatively high; a C17-position side chain is introduced by using tosylmethyl isocyanide, so that acetone cyanohydrin serving as a highly-toxic reagent is prevented from being used; and the synthesis method is safe, environment-friendly and suitable for industrial production.
Owner:山东国九堂制药集团股份有限公司
Cyclopropane alpha-amino acid derivatives containing continuous quaternary carbon center and synthesis method thereof
ActiveCN104910036AImprove adaptabilityWide adaptabilityOrganic compound preparationCarboxylic acid amides preparationChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis of drugs, and discloses cyclopropane alpha-amino acid derivatives containing continuous quaternary carbon center and a synthesis method thereof. The synthesis method comprises the following steps: adding N-p-tolylsulfonylone hydrazone, methyl 2-acetaminoacrylate, an alkali, a phase-transfer catalyst and a solvent into a reactor, and stirring at 70-90 DEG C to react for 12-24 hours; and after the reaction finishes, cooling to room temperature, filtering the reaction solution, distilling under reduced pressure to remove the solvent to obtain a crude product, and purifying by column chromatography to obtain the cyclopropane alpha-amino acid derivatives containing continuous quaternary carbon center. The method avoids using any transition metal catalyst, and uses the nontoxic, cheap and accessible raw materials. The method has the advantages of high reaction adaptability to functional groups, wide substrate adaptability, high product yield and favorable diastereoselectivity, can implement gram-scale production and synthesis, and is beneficial to industrial production. The obtained product has wide application range in synthesis of pesticides, drugs and natural products.
Owner:SOUTH CHINA UNIV OF TECH
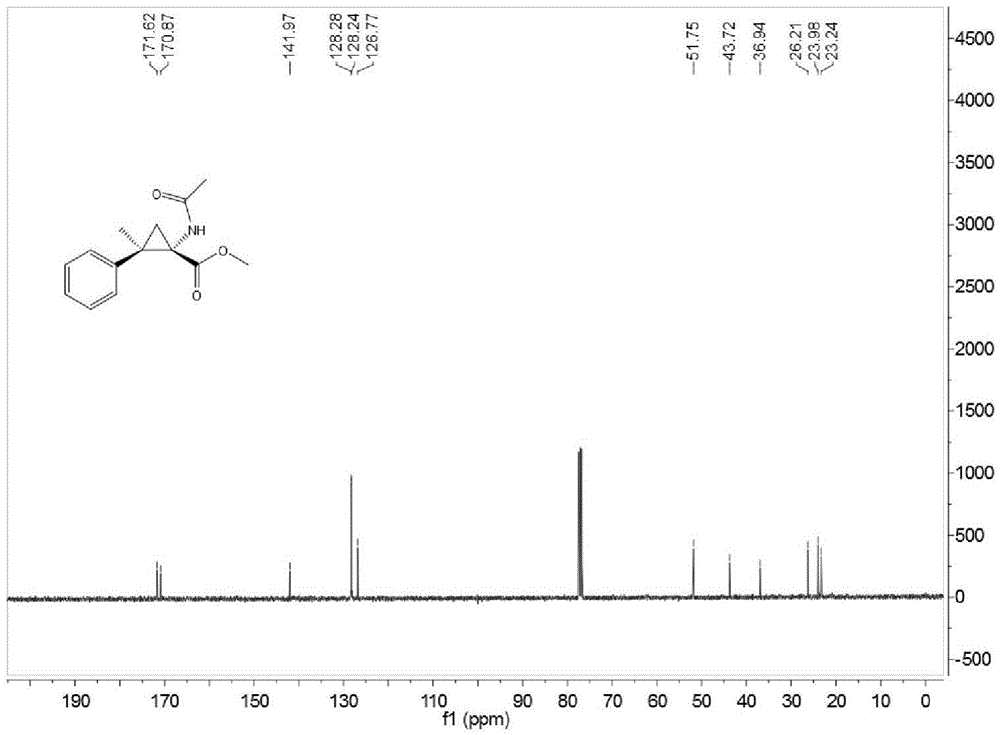
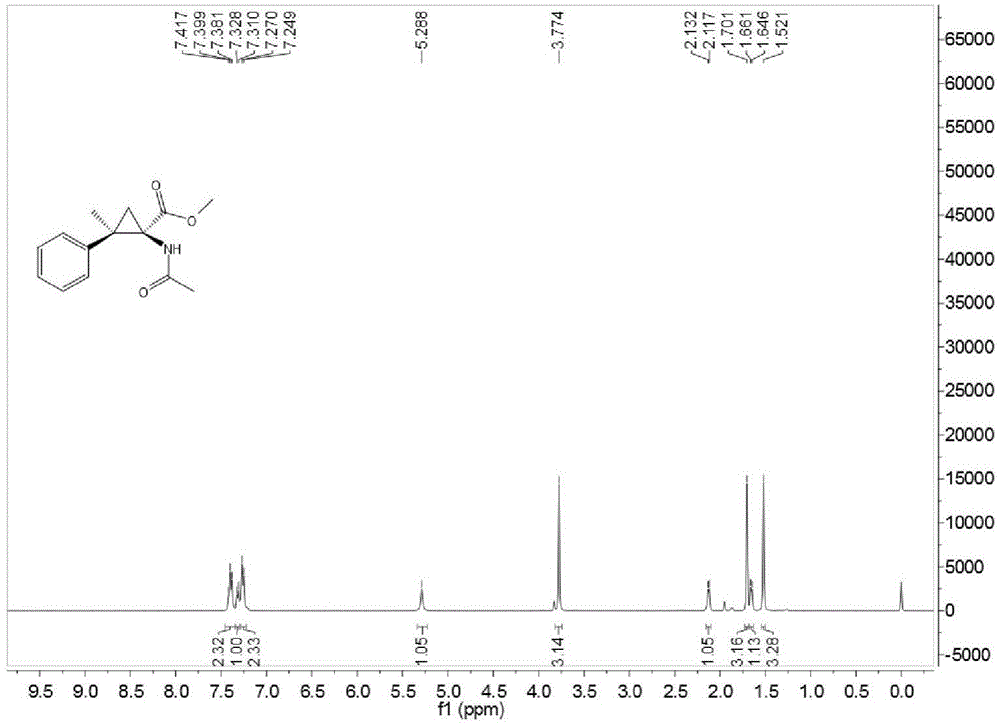
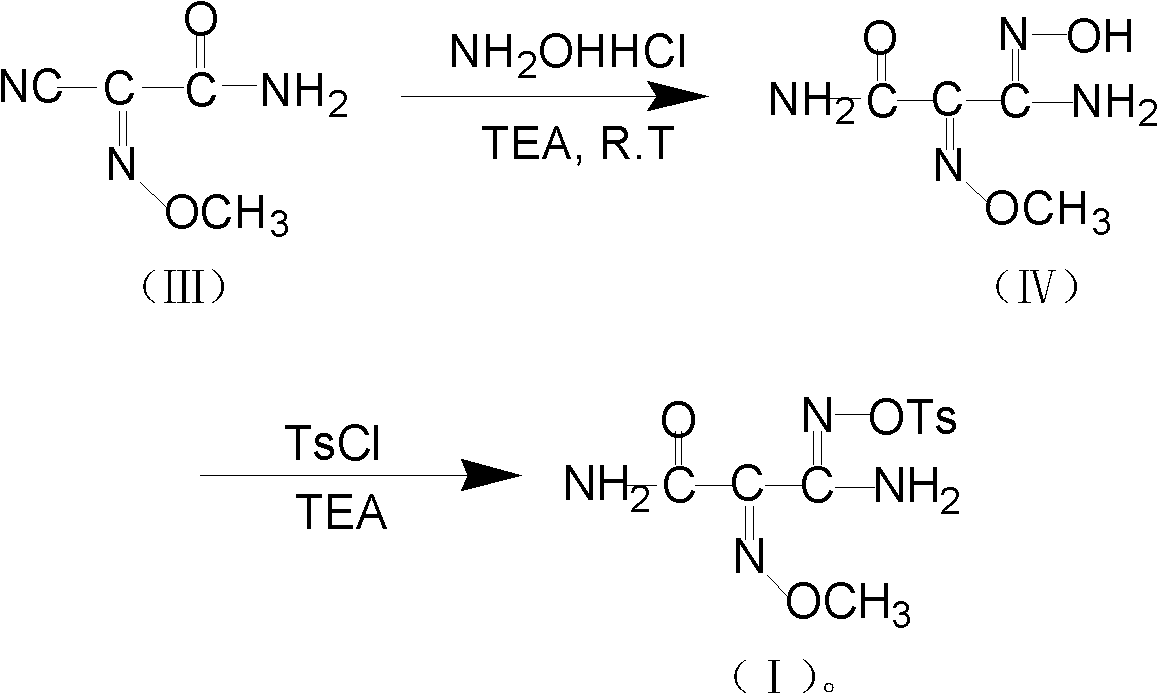
Method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime
ActiveCN102093266AShort processReduce manufacturing costSulfonic acid esters preparationSulfonyl chlorideReaction intermediate
The invention relates to a method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime, which comprises the following steps: (1) reacting cyanoacetamide, which is used as an initial raw material, with sodium nitrite in a nitrosation mode to obtain a reaction intermediate, and reacting the reaction intermediate with dimethyl sulfate in an esterification mode to obtain 2-cyano-2-methoxyl-imido-acetamide; and (2) under the action of hydroxylamine hydrochloride, hydrolyzing cyano groups in the 2-cyano-2-methoxyl-imido-acetamide obtained in the step (1) into amino groups, and reacting with p-methylbenzene sulfonyl chloride in an esterification mode to generate the O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime. The preparation method provided by the invention has the advantages of accessible raw materials, short technical process and high yield, and is suitable for industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
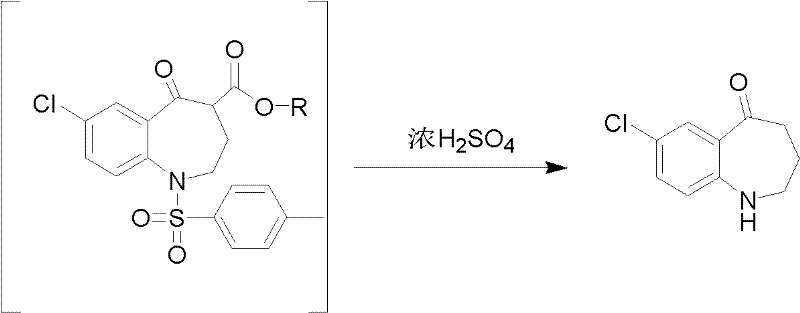
Preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine
The invention relates to a preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine. The 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine is an important intermediate for preparing arginine pitressin V2 receptor antagonist Tolvaptan. In the preparation method, the target product 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine is prepared by removing tosyl and carbalkoxy from 7-chlorine-5-oxo-4-carbalkoxy-1-tosyl-2,3,4,5-tetrahydro-1-benzoazepine serving as a raw material under the action of sulfuric acid and by a 'one-pot method'. The sulfuric acid is used for performing 'one-pot method' reaction, so reaction steps are reduced and reaction time is greatly shortened. The preparation method has the advantages of simpleness and convenience for operation, equipment and cost saving, simpleness and practicability in separation and purification, short reaction time, high yield and high product purity and is applicable to industrialized production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com