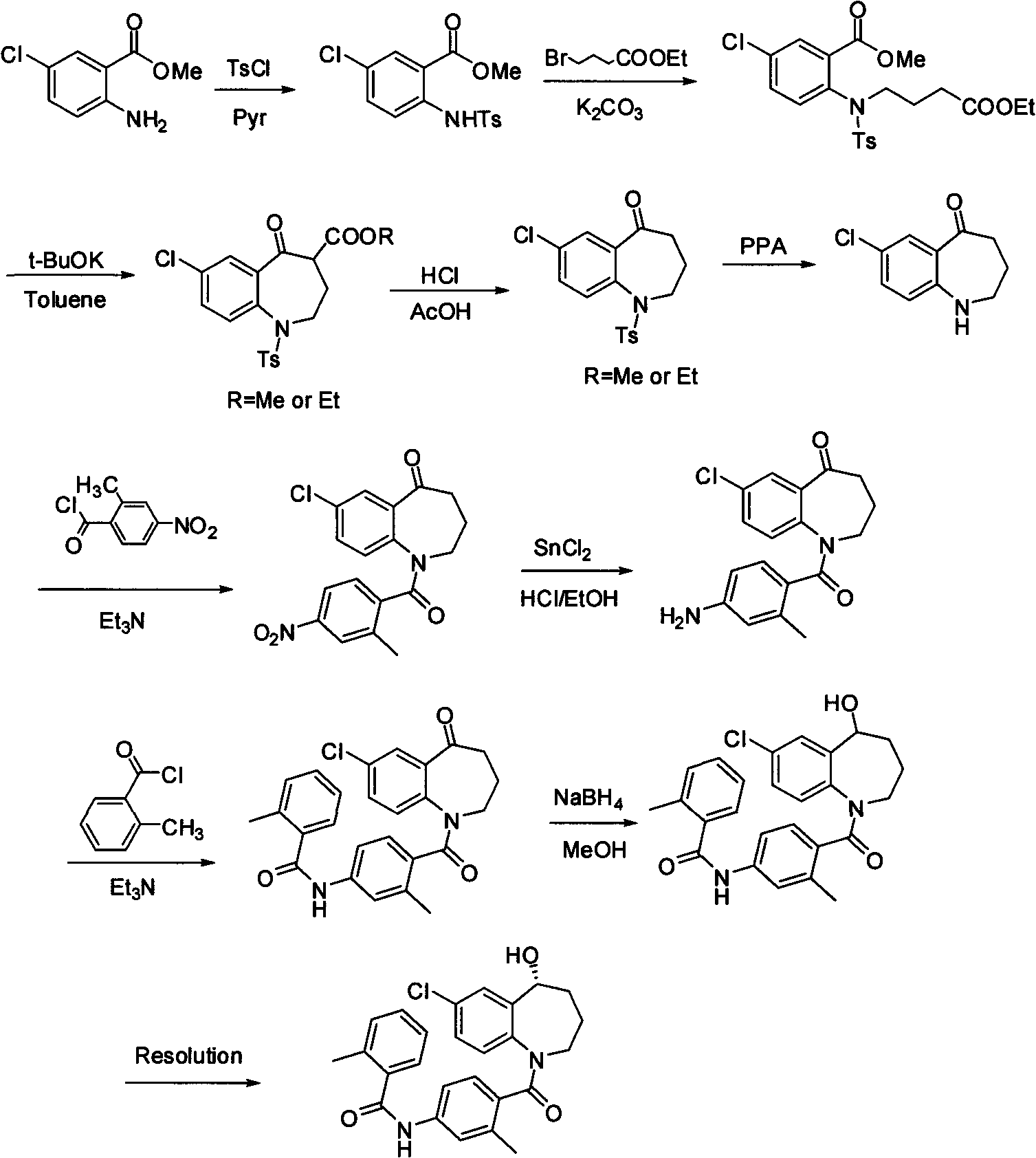
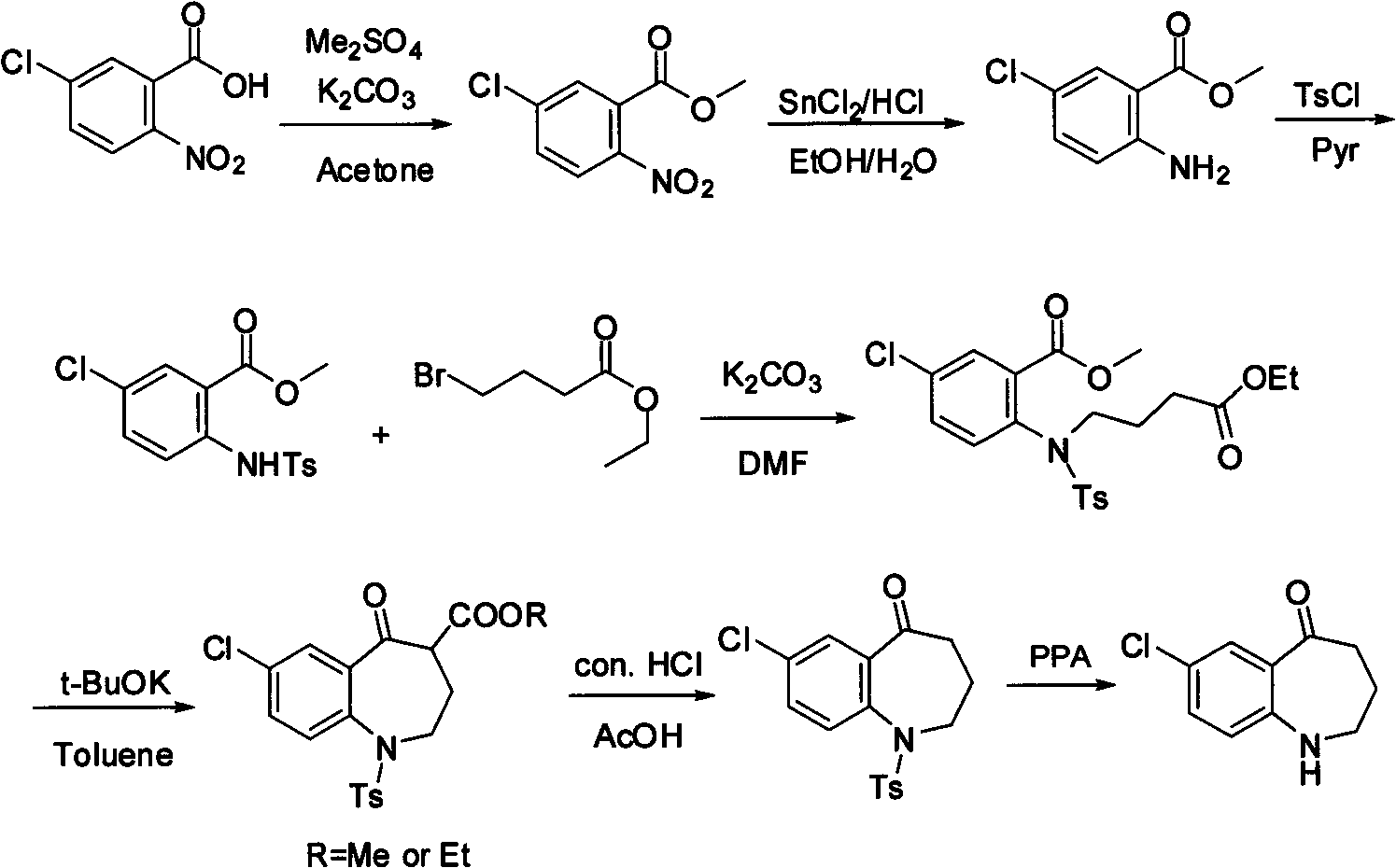
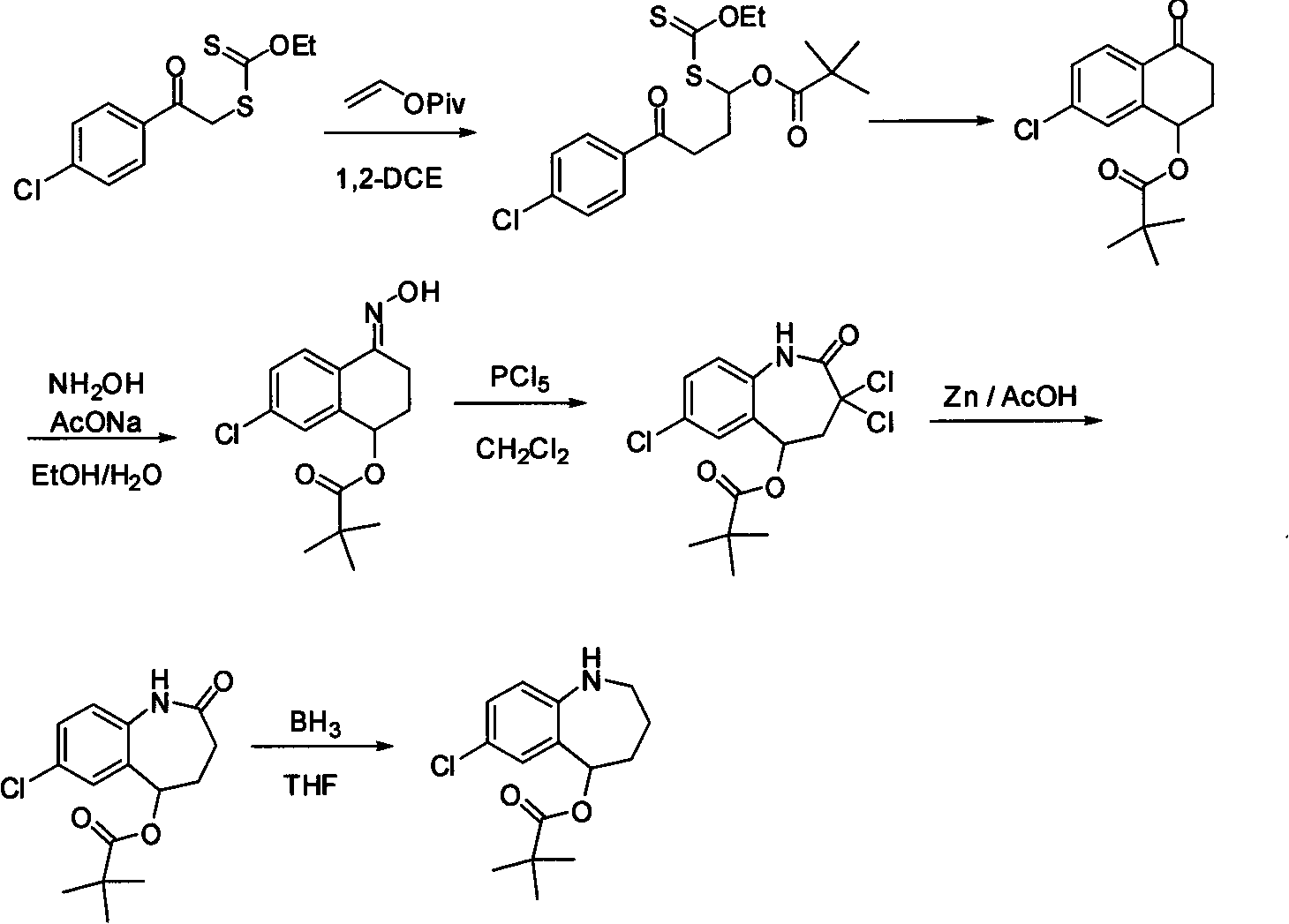
Method for preparing 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepino-5-ketone
A technology for benzoazepines and zebralines is applied in the field of preparation of benzoazepines, can solve the problems of high cost, low yield, and cannot be well applied to industrialized production, and achieves the effect of reducing industrial costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] p-Chloroaniline (89.3g, 0.70mol) and dichloromethane (250mL) were placed in a 2L reaction flask, and pyridine (60.9g, 62.3mL, 0.77mol) was added. The reaction solution was cooled to 0-5°C in an ice-water bath, and a solution of p-toluenesulfonyl chloride (139.2 g, 0.73 mol) and dichloromethane (250 mL) was added dropwise with stirring, and the drop was completed within 1 h. After dropping, the reaction was stirred at room temperature for 10 h. Water (500 mL) was added to the reaction solution, stirred for 10 min, and the layers were separated. The aqueous layer was extracted once with dichloromethane (100 mL). The organic layers were combined, washed with saturated brine (400 mL), and dried over anhydrous sodium sulfate. Dichloromethane was evaporated to dryness under reduced pressure, the resulting solid was added to methanol (400mL) and heated to dissolve, cooled to 0-5°C, slowly added water (400mL), kept stirring at 0-5°C for 30min, filtered, and cooled a little to...
Embodiment 2
[0039] p-Chloroaniline (89.3g, 0.70mol) and dichloromethane (250mL) were placed in a 2L reaction flask, and pyridine (55.4g, 56.6mL, 0.70mol) was added. The reaction solution was cooled to 0-5°C in an ice-water bath, and a solution of p-toluenesulfonyl chloride (160.2 g, 0.84 mol) and dichloromethane (250 mL) was added dropwise with stirring, and the drop was completed within 1 h. After dropping, the temperature was raised to dichloromethane reflux, and the reaction was stirred for 7h. Water (500 mL) was added to the reaction solution, stirred for 10 min, and the layers were separated. The aqueous layer was extracted once with dichloromethane (100 mL). The organic layers were combined, washed with saturated brine (400 mL), and dried over anhydrous sodium sulfate. Dichloromethane was evaporated to dryness under reduced pressure, the resulting solid was added to methanol (400mL) and heated to dissolve, cooled to 0-5°C, slowly added water (400mL), kept stirring at 0-5°C for 30m...
Embodiment 3
[0041] p-Chloroaniline (89.3g, 0.70mol) and dichloromethane (250mL) were placed in a 2L reaction flask, and pyridine (83.0g, 85.0mL, 1.05mol) was added. The reaction solution was cooled to 0-5°C in an ice-water bath, and a solution of p-toluenesulfonyl chloride (152.5 g, 0.80 mol) and dichloromethane (250 mL) was added dropwise with stirring, and the drop was completed within 1 h. After dropping, the temperature was raised to dichloromethane reflux, and the reaction was stirred for 5h. Water (500 mL) was added to the reaction solution, stirred for 10 min, and the layers were separated. The aqueous layer was extracted once with dichloromethane (100 mL). The organic layers were combined, washed with saturated brine (400 mL), and dried over anhydrous sodium sulfate. Dichloromethane was evaporated to dryness under reduced pressure, the resulting solid was added to methanol (400mL) and heated to dissolve, cooled to 0-5°C, slowly added water (400mL), kept stirring at 0-5°C for 30m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com