Polycation gene carrier as well as preparation method and application thereof
A gene carrier and polycation technology, applied in the field of biomedical new materials, can solve the problems of poor repeatability and complicated preparation methods, and achieve the effect of simple preparation process and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention provides a preparation method of the polycationic gene carrier described in the above technical solution, comprising the following steps:
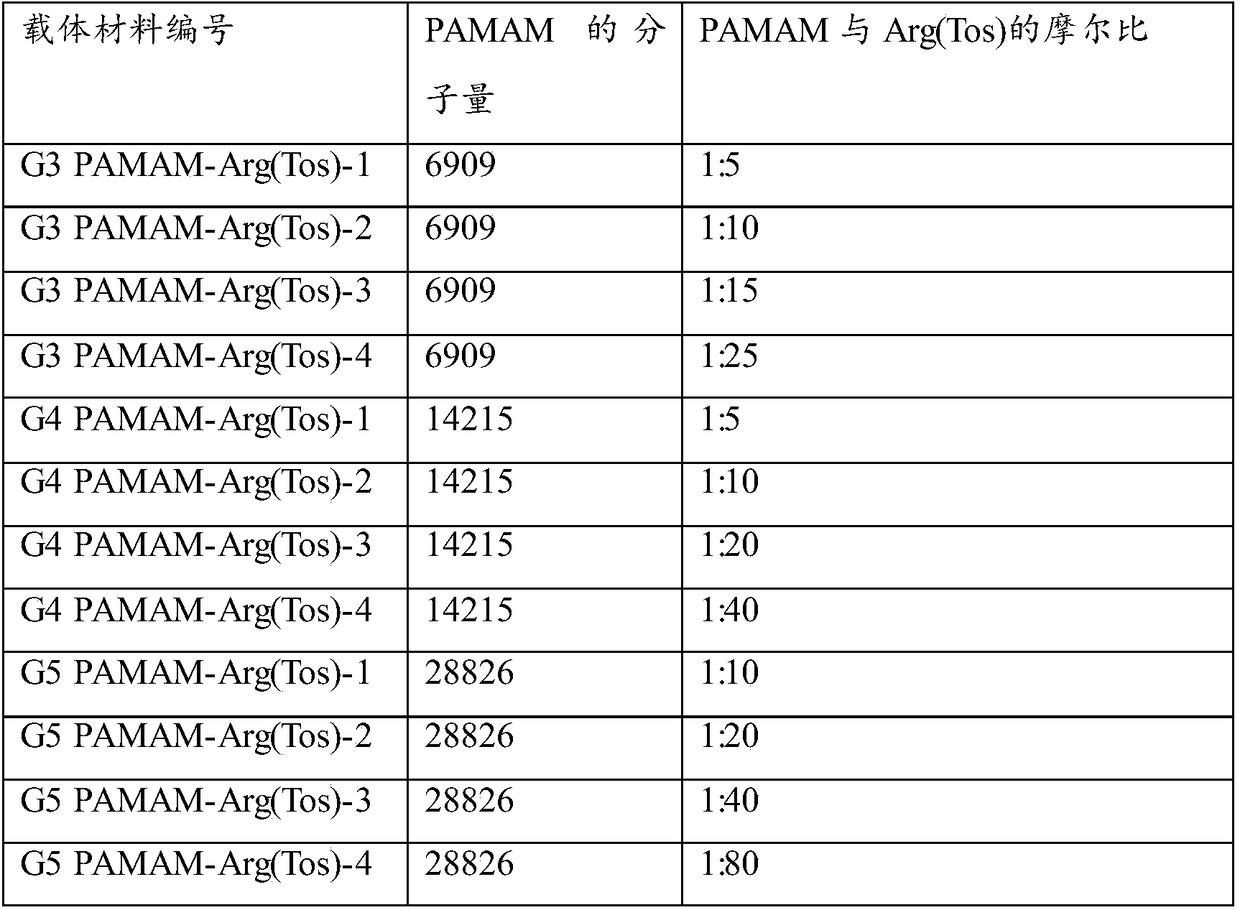
[0037] a) reacting the activated p-toluenesulfonyl and t-butoxycarbonyl double-protected arginine solution with the grafted skeleton raw material solution, dialyzed, and freeze-dried to obtain a freeze-dried product; the grafting in the grafted skeleton raw material solution The skeleton raw material is selected from dendritic polyamide-amine, hyperbranched polylysine with molecular weight of 2000-100000 g / mol or linear ε-polylysine with molecular weight of 2000-50000 g / mol; the dendritic polyamide-amine is selected from One or more of model PAMAM-G3, model PAMAM-G4 and model PAMAM-G5;
[0038] b) reacting the freeze-dried product with trifluoroacetic acid, and precipitating the reaction product to obtain a polycationic gene carrier.
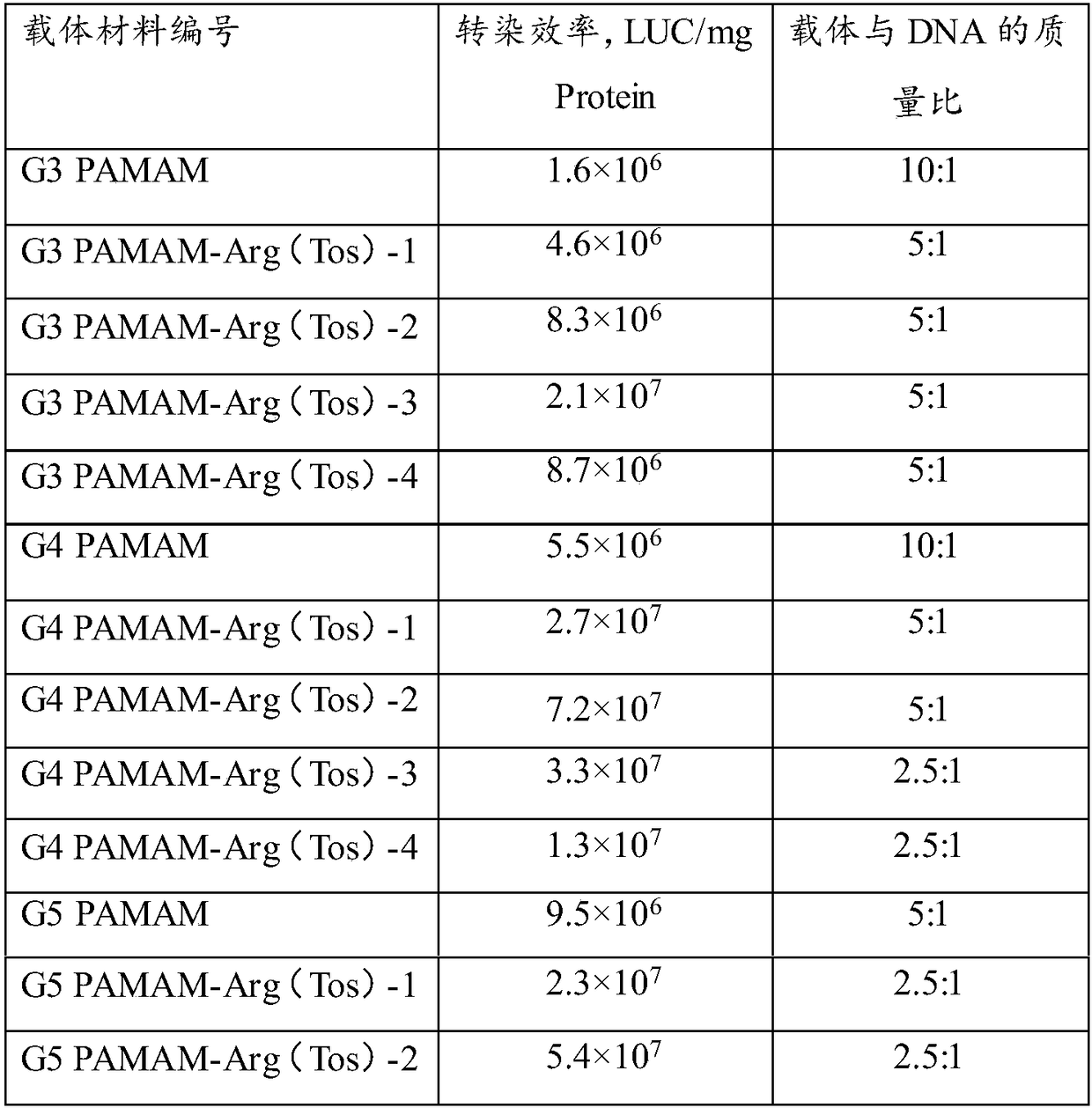
[0039] The preparation process is simple and reproducible, and plays an imp...
Embodiment 1
[0093] Example 1: Preparation of PAMAM-Arg (Tos)
[0094] (1) Synthesis of PAMAM-Arg(Tos) of G3 generation.
[0095] The PAMAM (200 mg, 0.0289 mmol) of the G3 generation was weighed and dissolved in 4 mL of anhydrous methanol, and a certain amount of p-toluenesulfonyl and tert-butoxycarbonyl double-protected arginine was added according to the designed molar charging ratio (see Table 1). (Boc-Arg(Tos)-OH) was dissolved in anhydrous methanol (dissolved according to 1 g of Boc-Arg(Tos)-OH / 10 mL of methanol). EDCI (2 times the molar equivalent of Boc-Arg(Tos)-OH) and HOBT (2 times the molar equivalent of Boc-Arg(Tos)-OH) were added to the Boc-Arg(Tos)-OH solution to activate the reaction After 1 h, the methanol solution of PAMAM was slowly added to the above mixture, and the reaction was carried out at room temperature for 4 days. The reaction mixture was dialyzed and lyophilized. Then, the lyophilized product was reacted under the condition of trifluoroacetic acid for 4 h, co...
Embodiment 2
[0103] Example 2: Preparation of HBPLL-Arg(Tos)
[0104] (1) Synthesis of HBPLL-Arg(Tos) of HBPLL group with molecular weight of 2000. The weighed HBPLL (200 mg, 0.1 mmol) was dissolved in 4 mL of anhydrous methanol, and a certain amount of Boc-Arg(Tos)-OH was dissolved in anhydrous methanol (according to 1 g Boc- Arg(Tos)-OH / 10mL methanol dissolved). EDCI (2 times the molar equivalent of Boc-Arg(Tos)-OH) and HOBT (2 times the molar equivalent of Boc-Arg(Tos)-OH) were added to the Boc-Arg(Tos)-OH solution at room temperature The activation reaction was carried out for 1 h, and then the methanol solution of PAMAM was slowly added to the above mixture, and the reaction was carried out at room temperature for 4 days. The reaction mixture was dialyzed and lyophilized. Then, the lyophilized product was reacted under the condition of trifluoroacetic acid for 4 h, concentrated in vacuo, precipitated with anhydrous ether, vacuum-dried, dialyzed, lyophilized to obtain a solid produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com