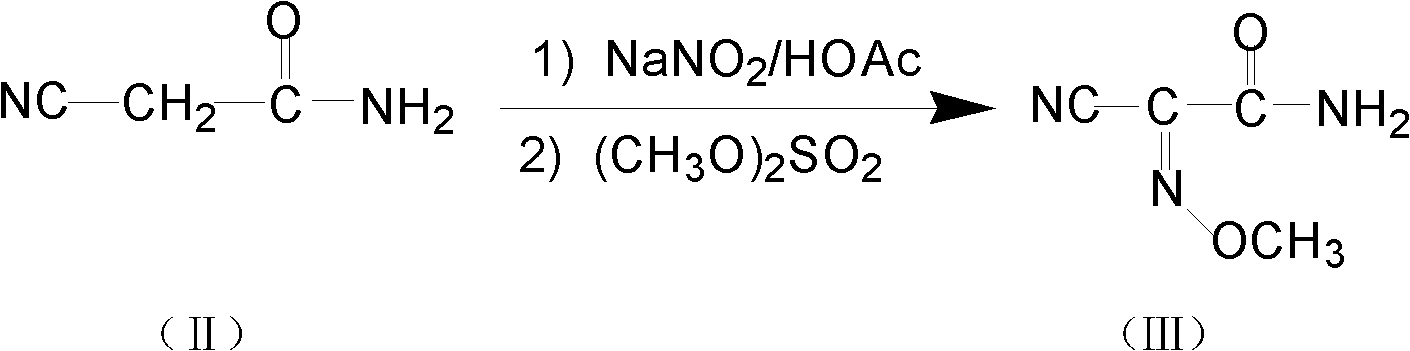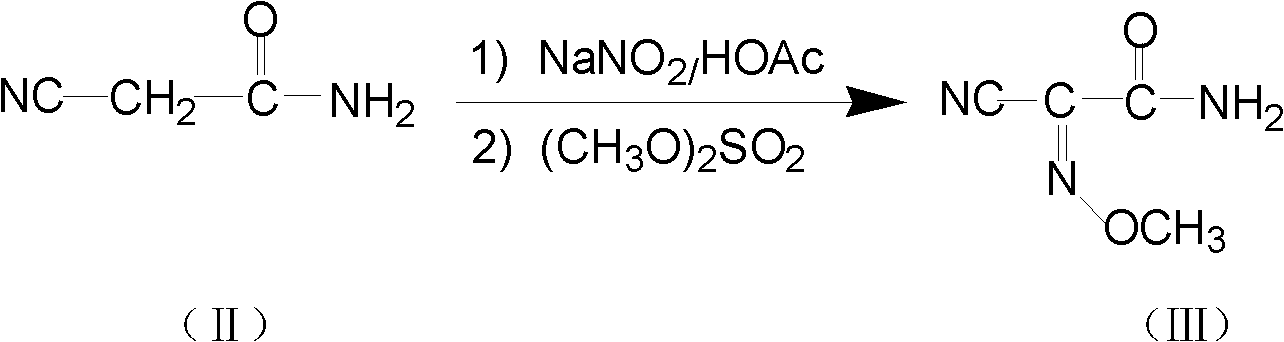Method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime
A technology of methoxyiminoacetamide oxime and methoxyiminoacetamide, which is applied in the field of organic synthesis, can solve the problems of high production cost, low yield, harsh reaction conditions, etc., so as to reduce production cost, shorten the process flow, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step: the preparation of intermediate 2-cyano-2-methoxyiminoacetamide (compound III)
[0031] Put 45g (0.53mol) of cyanoacetamide into a 500mL three-necked flask, 90mL of water, then drop in 36.6g (0.53mol) of sodium nitrite, control the temperature between 38-48°C, and then dropwise add sulfuric acid with a mass fraction of 62.5% 38g (0.23mol), stirred at the same temperature for 2-3h after the dropwise addition, TLC method monitoring reaction (developing agent is ethyl acetate: petroleum ether=5: 3) to the end, adding mass fraction is 40% sodium carbonate Adjust the pH of the reaction solution to 8.5-9.0, and add 67g (0.53mol) of dimethyl sulfate dropwise between 38-48°C with temperature control. After the dropwise addition, continue to stir for 3h, cool with ice water, filter the solid crystals, and use Wash with cold water and dry to obtain 50 g of 2-cyano-2-methoxyiminoacetamide, mp: 165-170°C. The liquid phase normalized content is 98.99%, and the yield...
Embodiment 2
[0033] The first step: the preparation of intermediate 2-cyano-2-methoxyiminoacetamide (compound III)
[0034] Put 45g (0.53mol) of cyanoacetamide into a 500mL three-necked flask, 90mL of water, then drop in 36.6g (0.53mol) of sodium nitrite, control the temperature between 38-48°C, and then dropwise add sulfuric acid with a mass fraction of 62.5% 50.0g (0.32mol), stirred at the same temperature for 2-3h after dropping, TLC method monitoring reaction (developing agent is ethyl acetate:petroleum ether=5:3) to the end, adding mass fraction is 10% hydrogen Sodium oxide solution, control the pH=7.5-8.5, control the temperature between 38-48°C, add 100.0g (0.79mol) of dimethyl sulfate dropwise, continue stirring for 3h after the dropwise addition, cool with ice water, and filter the solid crystal , washed with cold water and dried to obtain 45g of 2-cyano-2-methoxyiminoacetamide, mp: 165-170°C. The liquid phase normalized content is 98.5%, and the yield is 65.85%.
Embodiment 3
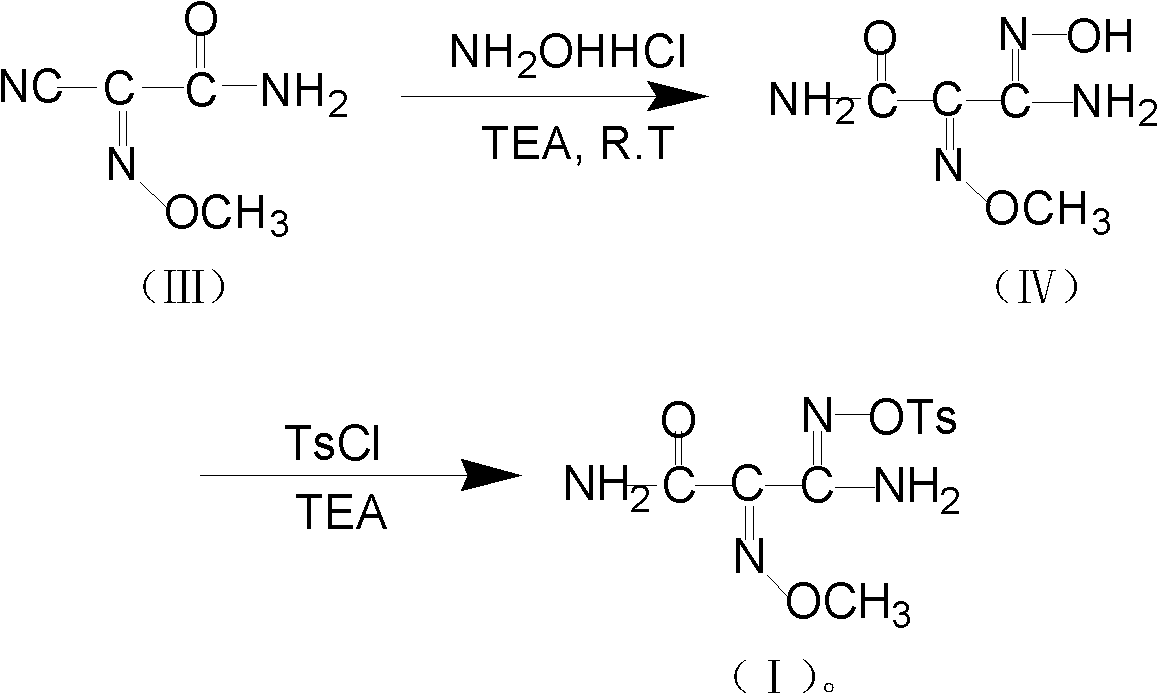
[0036] The second step: the preparation of O-tosyl-2-carbamoyl-2-methoxyiminoacetamide oxime (compound I)
[0037]Add 20g (0.16mol) 2-cyano-2-methoxyiminoacetamide, 12g (0.17mol) hydroxylamine hydrochloride, 160mL methanol to a 500mL three-necked flask, stir at room temperature, and slowly dropwise add 24mL (0.17 mol) triethylamine, stirred for about 5 hours after the addition, and the observation of the expansion plate showed that there was no raw material point (developing agent: methanol: petroleum ether = 5: 3), the reaction solution was cooled, and about 60mL of methanol was added, and 33.2g (0.17mol) p-toluenesulfonyl chloride, control the internal temperature below 5°C, add p-toluenesulfonyl chloride little by little, and drop 30mL (0.21mol) triethylamine at the same time, continue to Stir at high temperature for 1 hour, and the observation of the developing plate shows that there is no raw material point (developing agent is methanol:petroleum ether=5:3), then the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com