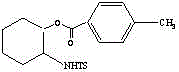
Method for performing ring-opening for cyclohexylaziridine by carboxylic acid
A technology of heterocyclopropane and cyclohexane nitrogen, applied in the field of organic synthesis, can solve problems such as large excess input of potassium acetate, and achieve the effects of reduced price, low price and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 50 mg of tosyl-activated cyclohexaneaziridine, 4.5 mg of KOH, 14 μL of acetic acid, and 1.0 mL of dimethyl sulfoxide into the test tube, heat to 35° C. and stir for 12 h. After the reaction, with 5% K 2 CO 3 The remaining acid was removed from the solution, and extracted three times with dichloromethane. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain a crude product. After the crude product was purified by silica gel chromatography, the ring-opened product A was obtained with a yield of 98%.
[0026]
[0027] NMR spectrometer: Bruker AVANCE III 600MHz, solvent CDCl 3 , internal standard TMS. 1 H NMR data were collected at 600MHz, chemical shifts are in ppm, where CDCl 3 chemical shift( δ ) 7.27, coupling constant unit Hz; 13 C NMR data were collected at 150MHz under fully decoupled conditions, where CDCl 3 chemical shift( δ ) 77.0. ...
Embodiment 2
[0030] Add 50 mg of tosyl-activated cyclohexaneaziridine, 4.5 mg of KOH, 9 μL of formic acid, and 1.5 mL of N-methylpyrrolidone into the test tube, heat to 45° C. and stir for 4 h. After the reaction, with 5% K 2 CO 3The remaining acid was removed from the solution, and extracted three times with dichloromethane. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain a crude product. After the crude product was purified by silica gel chromatography, the ring-opened product B was obtained with a yield of 64%.
[0031]
[0032] 1 H NMR (600MHz, CDCl 3 ): δ 1.11-1.24 (m, 4H), 1.66-1.70 (m, 2H), 2.00-2.02 (m, 2H), 2.42 (s, 3H), 3.23-3.28 (m, 1H), 4.64-4.68 ( td, J=10.2, 5.4Hz, 1H), 4.90 (m, 1H), 7.28-7.29 (d, J=8.4Hz, 2H), 7.53 (s, 1H), 7.73-7.74 (d, J=7.8Hz , 2H )ppm.
Embodiment 3
[0034] Add 50 mg of tosyl-activated cyclohexaneaziridine, 4 mg of NaOH, 36.6 mg of benzoic acid, and 1.2 mL of dimethyl sulfoxide into the test tube, heat to 55° C. and stir for 2 h. After the reaction, with 5% K 2 CO 3 The remaining acid was removed from the solution, and extracted three times with dichloromethane. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain a crude product. After the crude product was purified by silica gel chromatography, the ring-opened product C was obtained with a yield of 98%.
[0035]
[0036] 1 H NMR (600MHz, CDCl 3 ): δ 1.28-1.48 (m, 4H), 1.69-1.76 (m, 3H), 2.00-2.04 (m, 1H), 2.18 (s, 3H), 2.22-2.24 (m, 1H), 3.31-3.32 ( m, 1H), 4.79-4.84 (td, J=10.2, 4.2 Hz, 1H), 5.04-5.05 (d, J=7.2, 1H), 6.90-6.92 (d, J=7.8, 2H), 7.35-7.38 (t, J=7.8 Hz, 2H), 7.53-7.58 (m, 3H), 7.75-7.76 (m, 2H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com