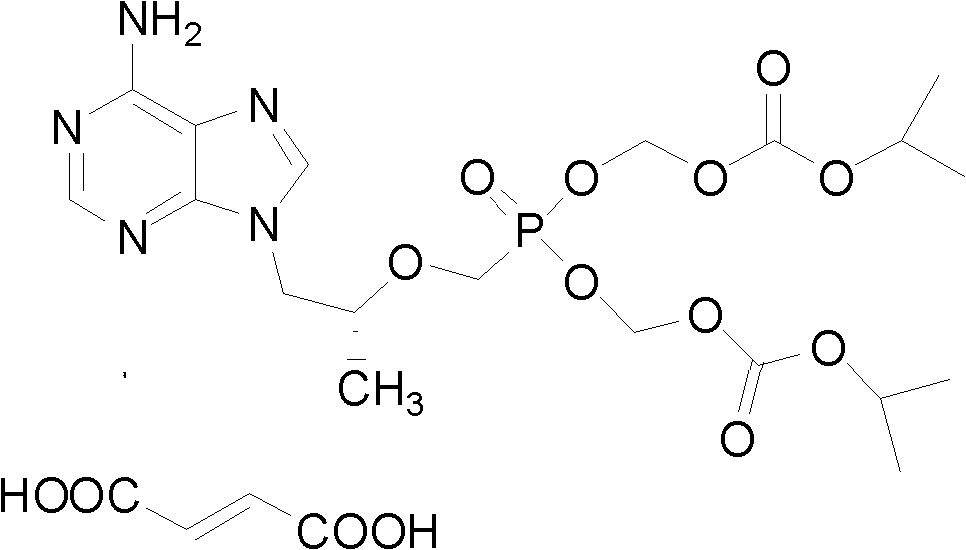
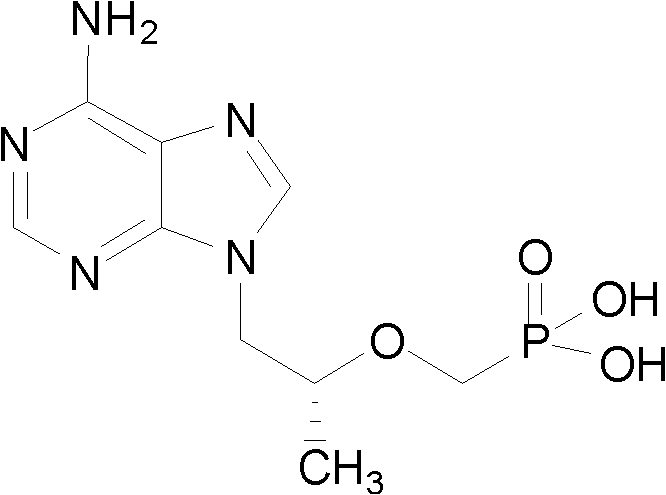
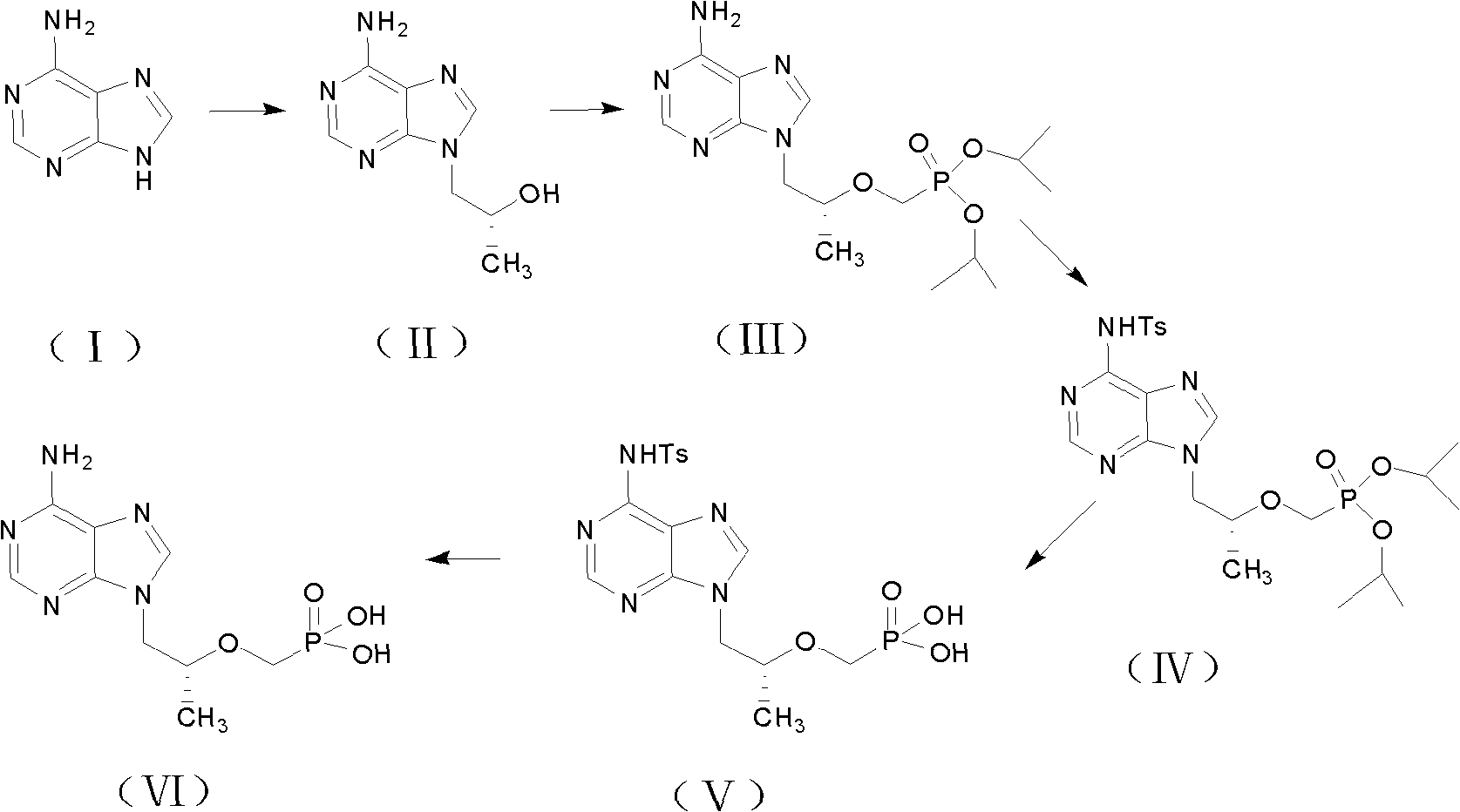
Preparation method for tenofovir
A technology of tenofovir and adenine, which is applied in the field of tenofovir preparation, can solve the problems of high cost and price, and achieve the effects of less three wastes, mild reaction conditions and improved product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In a 250ml reaction bottle, add adenine 20.0gDMF150ml at room temperature under nitrogen protection, further add (R)-propylene carbonate 18.0g, KOH 0.5g, heat up to 130°C for 8 hours, cool down to 70°C and add 200ml at a constant speed Stir the toluene at a low speed to crystallize, cool to room temperature and continue to cool down to 0-5°C, filter, wash with 50ml of frozen toluene, and dry the filter cake at 60°C to obtain 25.4g of (R)-9-(2-hydroxypropyl)adenine , Molar yield 89%.
[0050] In a 250ml reaction bottle, add 50ml of DMF at room temperature and under the protection of nitrogen, add 25.4g of the product from the previous step, add 22.4g of potassium tert-butoxide at room temperature, and slowly add 45.0g of diethyl p-toluenesulfonyloxyphosphonate dropwise after keeping for one hour React for 3 to 5 hours. After the reaction is complete, add 15.0 g of acetic acid to neutralize excess potassium alkoxide. After distilling DMF under reduced pressure, add 300 ml...
Embodiment 2
[0054] In a 250ml reaction bottle, add adenine 20.0g DMF150mL at room temperature under nitrogen protection, further add (R)-propylene carbonate 20.0g, NaOH 0.5g, heat up to 133°C for 5 hours, cool down to 70°C and add at a constant speed Stir 200ml of toluene at a low speed to crystallize, cool to room temperature and continue cooling to 0-5°C, filter, wash the filter cake with 50ml of frozen toluene, and blow dry at 60°C to obtain (R)-9-(2-hydroxypropyl)adenine 25.8 g, molar yield 90%.
[0055] In a 250ml reaction bottle, add 80ml of DMF at room temperature and under nitrogen protection, add 25.8g of the product from the previous step, add 24.0g of potassium tert-butoxide at room temperature, keep it for one hour, then add p-toluenesulfonyloxyphosphonic acid within 1 to 2 hours 50.0 g of diethyl ester was reacted for 2 to 3 hours. After the reaction was complete, 12.0 g of formic acid was added to neutralize excess potassium alkoxide. After the DMF was distilled under reduc...
Embodiment 3
[0058] In a 250ml reaction bottle, add adenine 20.0g DHF100ml at room temperature and under the protection of nitrogen, further add (R)-propylene carbonate 20.0g, KOH 0.5g, heat up to 136°C for 5 hours, cool down to 80°C and add at a constant speed 200ml of toluene was stirred at a low speed to crystallize, cooled to room temperature and then cooled to 0-5°C, filtered, washed with 50ml of frozen toluene, and the filter cake was blown-dried at 60°C to obtain (R)-9-(2-hydroxypropyl)adenine 24.5 g, molar yield 86%.
[0059] In a 250ml reaction bottle, add 50ml of DHF at room temperature and under the protection of nitrogen, add 24.5g of the product from the previous step, add 22.4g of potassium tert-butoxide at room temperature, keep it for one hour, and slowly add 45.0 g of diethyl p-toluenesulfonyloxyphosphonate g reaction for 3 to 5 hours, after the reaction is complete, add 15.0 g of propionic acid to neutralize excess potassium alkoxide, distill DHF under reduced pressure an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com