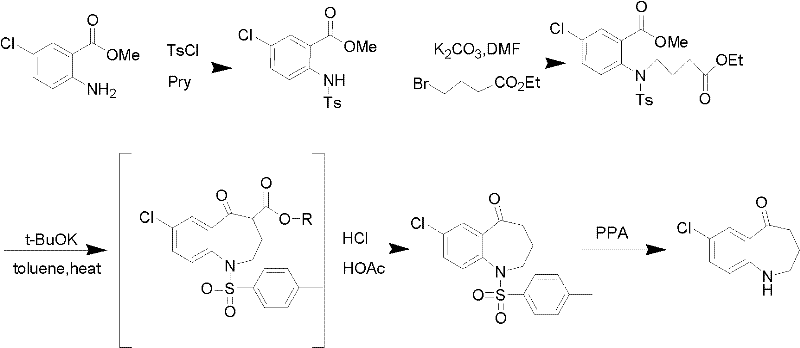
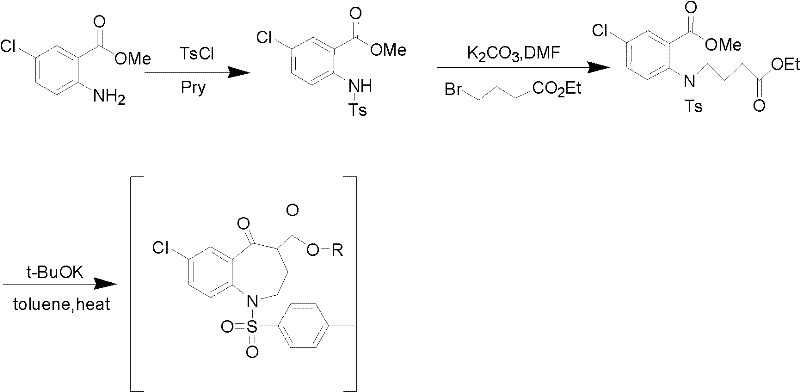
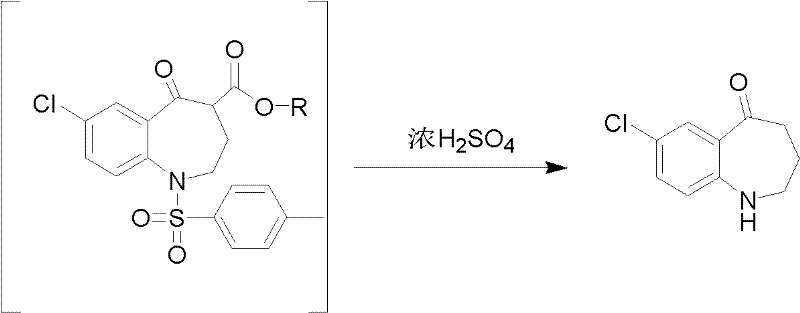
Preparation method for 7-chlorine-5-oxo-2,3,4,5-tetrahydro-1H-1-benzoazepine
A technology of benzonitrogen and oxo substitution, applied in the direction of organic chemistry, can solve the problems of long reaction route, easy moisture absorption cost, many impurities, etc., and achieve the effect of simplifying the post-processing method, shortening the reaction time and simplifying the reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Take 98% concentrated sulfuric acid (14.15ml) and dilute it to 85%, add it into a three-necked flask, stir for 10min under ice bath, get 7-chloro-5-oxo-4-alkoxycarbonyl-1-p-toluenesulfonyl- 2,3,4,5-Tetrahydro-1-benzazepine (2.87g, 0.0065mol) was added to the reaction flask, and after stirring for 15 minutes, the reaction system was removed from the ice bath and heated for reaction. The temperature of the reaction system was controlled at 90 ℃, after 1 hour of reaction, TLC detects that the reaction is over, stop the reaction, cool to room temperature, adjust the pH of the reaction system to 7-8 with NaOH solution (1mol / L) in an ice bath, and vacuum filter to obtain the dark yellow solid target product 7-chloro -5-Oxo-2,3,4,5-tetrahydro-1H-1-benzazepine (1.47 g, 99%), purity 99% (HPLC, normalization method).
Embodiment 2
[0019] Take 98% concentrated sulfuric acid (14.15ml), add it to a three-necked flask, stir for 10min under ice bath, get 7-chloro-5-oxo-4-alkoxycarbonyl-1-p-toluenesulfonyl-2,3, 4,5-tetrahydro-1-benzazepine (2.30g, 0.0052mol) was added to the reaction flask, and after stirring for 15 minutes, the reaction system was removed from the ice bath, 30ml of distilled water was added to dilute concentrated sulfuric acid, and the reaction was carried out at room temperature. After 0.5h, TLC detects that the reaction is over, stop the reaction, cool to room temperature, adjust the pH of the reaction system to 9-10 with KOH solution (1mol / L) in an ice bath, and vacuum filter to obtain the dark yellow solid target product 7-chloro-5 -Oxo-2,3,4,5-tetrahydro-1H-1-benzazepine (1.16 g, 97%), purity 98.5% (HPLC, normalization method).
Embodiment 3
[0021] Take 98% concentrated sulfuric acid (14.15ml) and dilute it to 50%, add it into a three-necked flask, stir for 10min under ice bath, get 7-chloro-5-oxo-4-alkoxycarbonyl-1-p-toluenesulfonyl- 2,3,4,5-Tetrahydro-1-benzazepine (3.28g, 0.0074mol) was added to the reaction flask, and after stirring for 15 minutes, the reaction system was removed from the ice bath and heated for reaction. The temperature of the reaction system was controlled at 100 ℃, after 5 hours of reaction, TLC detects that the reaction is over, stop the reaction, cool to room temperature, and use Na 2 CO 3 solution (10mol / L) to adjust the pH of the reaction system to 7-8, vacuum filtration to obtain the dark yellow solid target product 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzo Azepine (1.66 g, 98%), purity 98% (HPLC, normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com