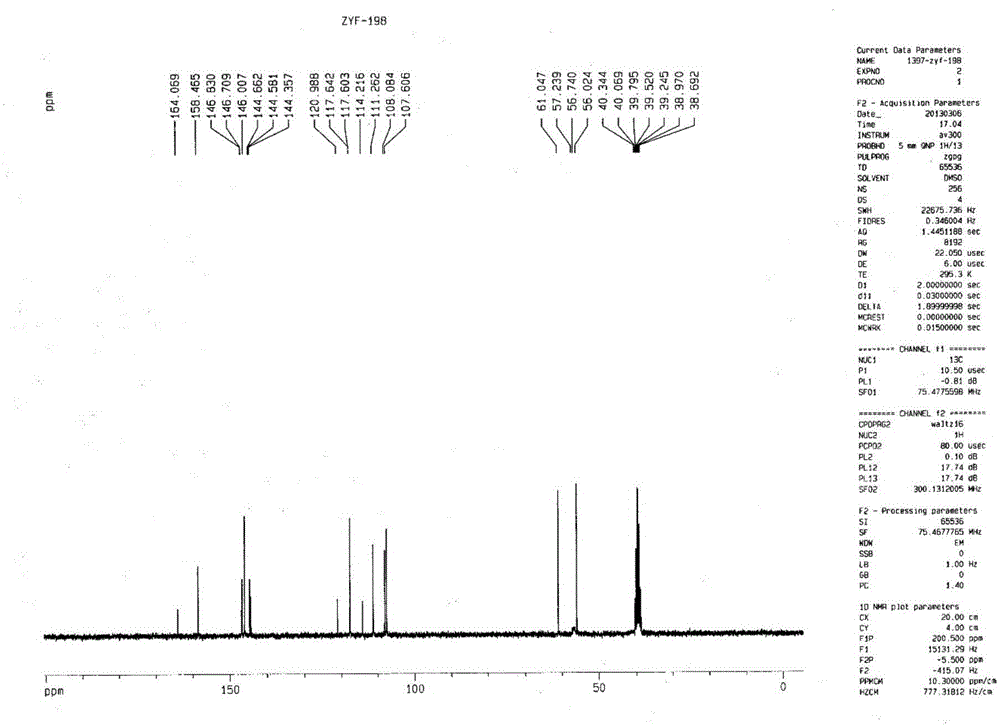
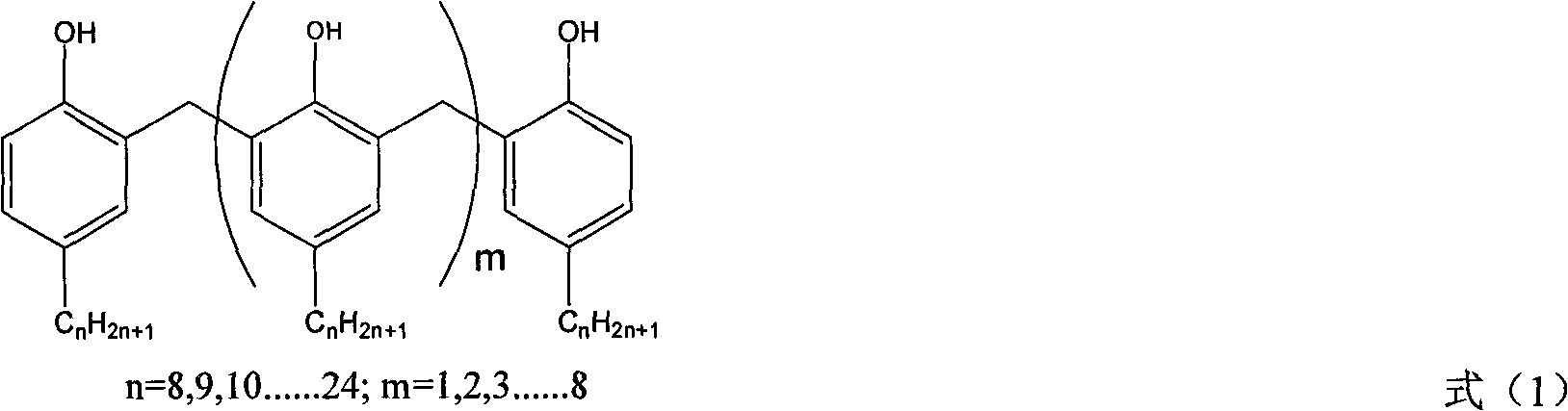
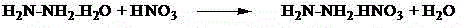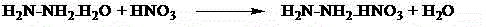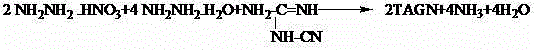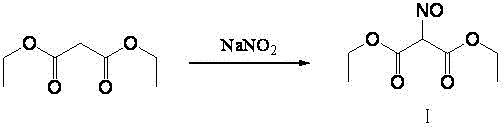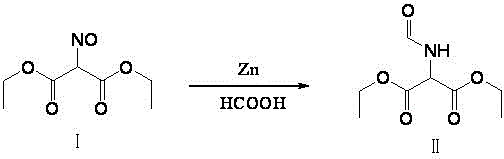Patents
Literature
121results about How to "The post-processing method is simple" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High-efficiency and green method for preparing pymetrozine
ActiveCN103724327AReduce manufacturing costPrevent the occurrenceOrganic chemistryWastewaterEthyl ester
The invention discloses a high-efficiency and green method for preparing pymetrozine. According to the method, a byproduct methyl acetate produced in a pymetrozine condensation step serves as a raw material and replaces ethyl acetate in a traditional process for synthesizing acethydrazide, and the produced byproduct methanol can serve as a solvent in a subsequent step, so that the byproduct is recycled, and the raw material utilization rate is improved. Hydrogen chloride or concentrated hydrochloric acid in a traditional process is replaced by adopting a saturated hydrogen chloride methanol solution in the condensation step, and moisture in the system is avoided, so that amino triazone is subjected to a hydrolysis reaction, and byproducts are basically eliminated. According to the method, the yield of the product and the utilization rate of the hydrogen chloride are improved, the reaction time is shortened, emission of wastewater and waste gas is reduced, the comprehensive production cost is reduced, and better conditions are created for industrial large-scale production of the product.
Owner:JIANGSU ANPON ELECTROCHEM
Synthetic method of polycarbosilane
InactiveCN105566637AImprove thermal stabilityThe post-processing method is simpleHeat stabilityHigh pressure
The invention discloses a synthetic method of polycarbosilane, and relates to a method for preparing polycarbosilane by using polydimethylsilane as a raw material. The invention aims to solve the technical problems that the yield of polycarbosilane is low and the heat stability is poor in the prior art. The method comprises a first step of adding polydimethylsilane and iron powder into an autoclave, replacing carbon dioxide gas 3 to 6 times in the autoclave, heating, raising the temperature within one hour and maintaining the temperature, heating again after reducing the temperature, maintaining the temperature, and cooling to a room temperature; a second step of dissolving a product obtained in the first step into a certain amount of xylene, filtering to obtain a clear and transparent solution, adding the same volume of ethanol solution, separating out a claybank solid product so as to obtain the polycarbosilane. According to the method disclosed by the invention, the polydimethylsilane is transformed into polycarbosilane at a high temperature and high pressure; the obtained polycarbosilane is narrow in molecular weight distribution and good in heat stability. The yield of the polycarbosilane can reach 52.8 percent. The invention belongs to the synthetic field of polycarbosilane.
Owner:INST OF PETROCHEM HEILONGJIANG ACADEMY OF SCI
Preparation method of edoxaban
ActiveCN109942600AStable structureHigh yieldOrganic chemistryBulk chemical productionSulfurN-Butyllithium
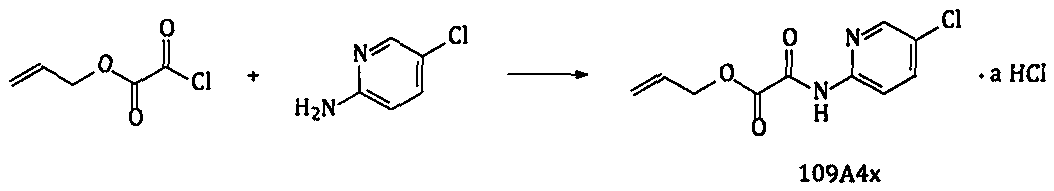
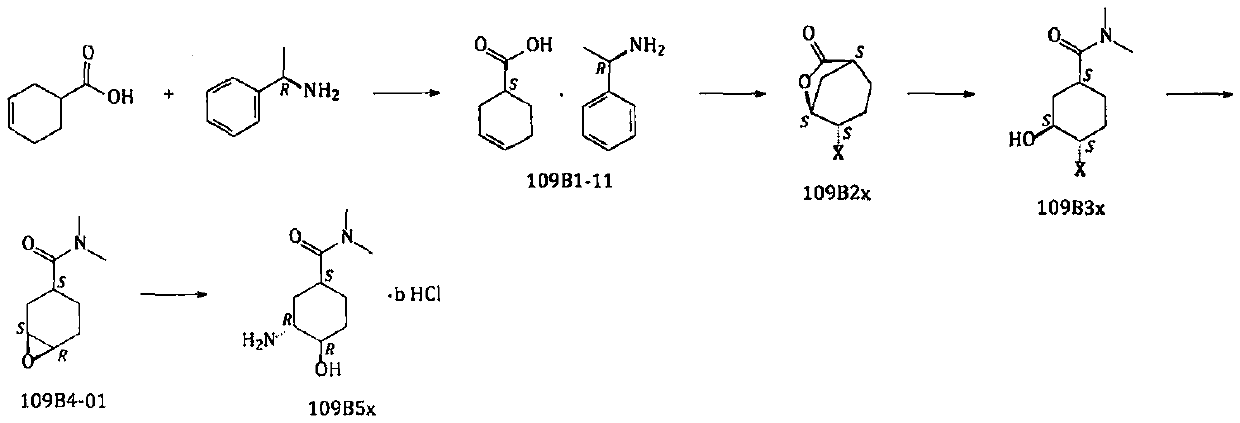
The invention relates to a new preparation route and a new method for a p-toluenesulfonic acid edoxaban hydrate and intermediates thereof. The new method comprises the steps that a high-reactivity compound 109A4x is prepared; a compound 109C6x is prepared by using a new synthesizing method; new compounds 109E8-01, 109E9x and 109T7-01 are prepared; the p-toluenesulfonic acid edoxaban hydrate is prepared by using the intermediates. By using the new method and the new route, the reaction step of copious cooling is omitted, and dangerous elemental sulfur, high-risk n-butyllithium and high-risk azides are prevented from being used. In a word, by means of the method, the p-toluenesulfonic acid edoxaban hydrate and the key intermediates thereof are more easily and safely prepared at a lower coston an industrialization scale.
Owner:内蒙古京东药业有限公司 +1
Preparation method of L-alanyl-L-glutamine
The invention relates to a preparation method of L-alanyl-L-glutamine; the preparation method comprises the following steps of: a) adding a solvent to a reaction container; stirring the solvent; and then adding N-phthaloyl-L-alanyl-L-glutamine to the reaction container; putting the reaction container in an ice bath; dripping a short-chain amine solution into a solution for clarification; removing the ice bath; adding the rest short-chain amine solution to the solution; after finishing dripping, carrying out room temperature reaction on the solution; decompressing and concentrating the solution to remove excessive short-chain amine in a water bath until the pH value of the solution is 6-7; standing for crystallization; filtering crystals; and drying filter cakes at a normal pressure so as to obtain a crude product; and b) adding water to the crude product at room temperature; dissolving the crude product in the water; dripping short-chain alcohol into the water for crystallization and purification during stirring, so as to obtain the L-alanyl-L-glutamine. Through the adoption of the preparation method of the L-alanyl-L-glutamine provided by the invention, hypertoxic hydrazine hydrate is avoided; and the preparation method of the L-alanyl-L-glutamine provided by the invention has the advantages of greater safety in operation, high product purity, high optical rotation and low impurity content.
Owner:山东诚汇双达药业有限公司
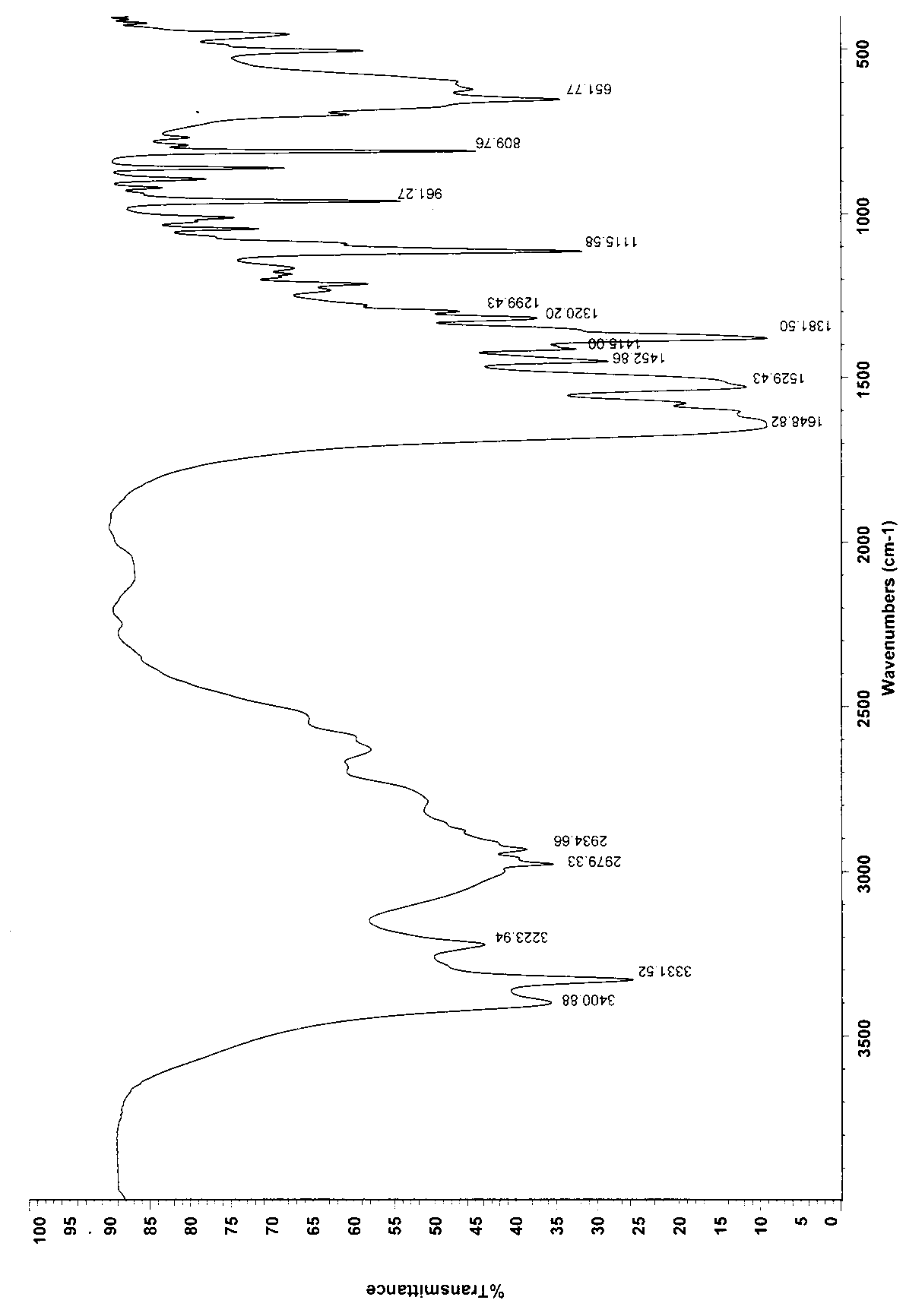
Method for synthesizing bis(2,4,4-trimethylpentyl) phosphinic acid with double initiators
InactiveCN102268038AShort reaction timeImprove conversion rateGroup 5/15 element organic compoundsSodium Hypophosphite MonohydrateSodium phosphates
The invention relates to a method for synthesizing bis(2,4,4-trimethylpentyl) phosphinic acid. The method adopts sodium hypophosphite and diisobutylene as the raw materials, and employs 2, 2'-azobis(2-methylpropionamidine) dihydrochloride and hydrogen peroxide as the radical initiators. With the steps of heating under violent stirring, a period of reaction at a constant temperature, liquid separation, organic phase washing, and vacuum distillation, the object product bis(2,4,4-trimethylpentyl) phosphinic acid can be obtained. The method provided in the invention has the advantages of short reaction time, high conversion rate, simple post-treatment method and atmospheric reaction, etc.
Owner:JIANGXI SCI & TECH NORMAL UNIV
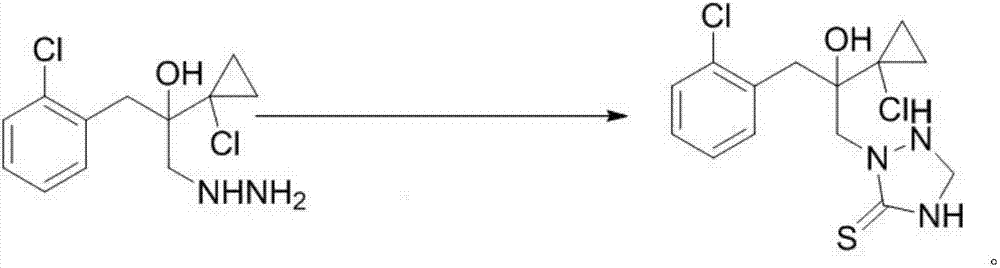
Preparation method of prothioconazole intermediate
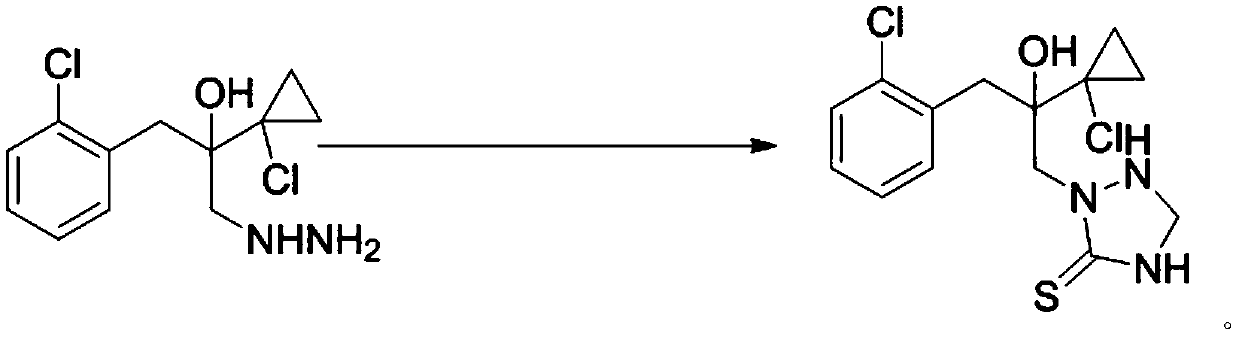
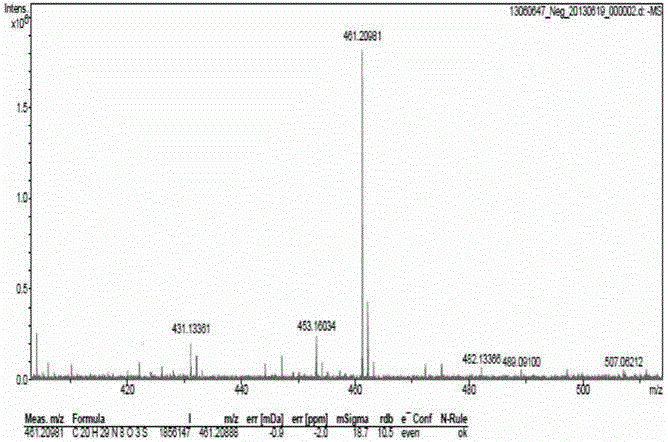
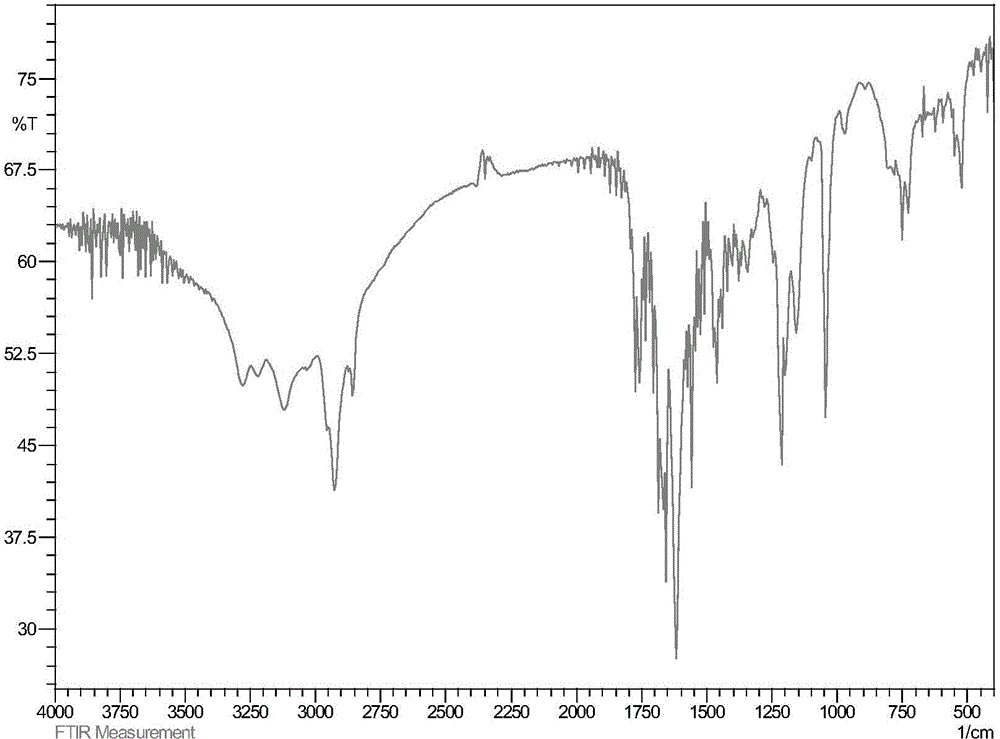
ActiveCN107445909AThe post-processing method is simpleShort reaction timeOrganic chemistryThioketoneHydrazine compound
The invention relates to a preparation method of a prothioconazole intermediate. The preparation method comprises the following steps: dissolving [1-(2-chlorophenyl)-2-(1-chlorocyclopropyl)-2-hydroxy]-propyl hydrazine into methyl benzene; dropwise adding a formaldehyde aqueous solution or a polyformaldehyde aqueous solution under 30 to 35 DEG C; then adding in thiocyanate after completion of dropwise adding; then dropwise adding hydrochloric acid or sulfuric acid aqueous solution; keeping warm and reacting for 50 to 70 minutes after completion of dropwise adding; directly using a reaction solution for follow-up reaction or filtering the reaction solution after cooling; thus obtaining the prothioconazole intermediate 2-(1-chloro-cyclopropyl-1-yl)-1-(2-chlorophenyl)-2-hydroxy-3-(1,2,4-triazolidine-5-thioketone-1-yl)-propane. Through optimization on a whole route, for example, reaction temperature, reagents, a feeding procedure and the like, of the preparation method, an after-treatment method is simple, three wastes such as waste gas, waste water and waste residues are few, the reaction time is shortened, the purity and the yield of a product are higher, and the preparation method is suitable for industrial production.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
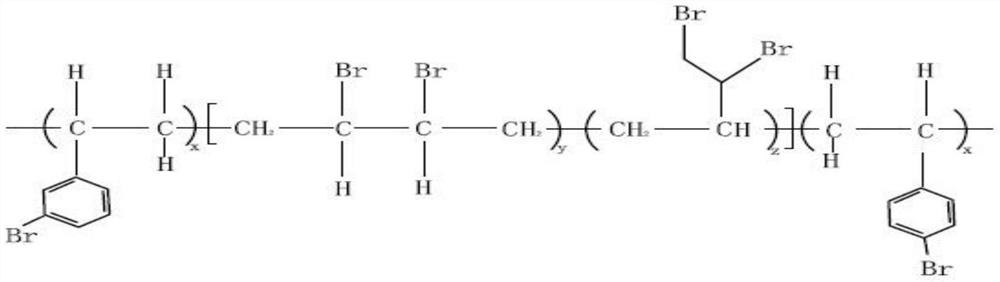
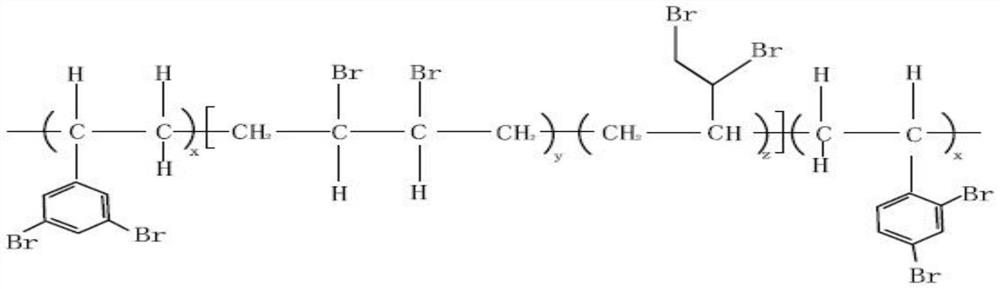
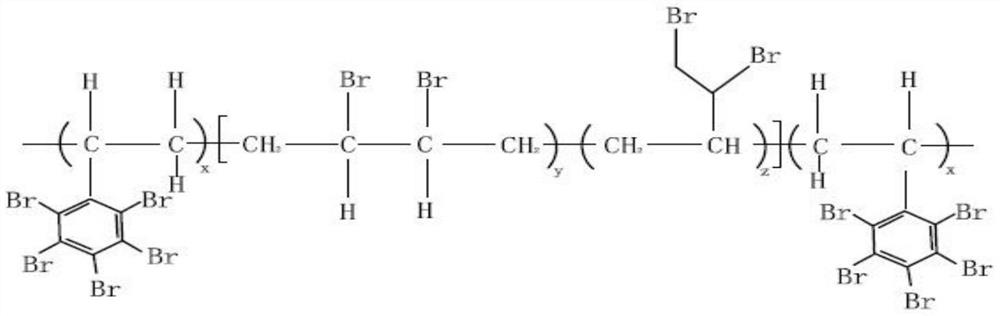
Aromatic and aliphatic chain co-brominated high-molecular polymer and preparation method thereof
The invention relates to an aromatic and aliphatic chain co-brominated high-molecular polymer and a preparation method thereof, and solves the technical problems of low product molecular weight, insufficient bromine content, poorer product performance, complex synthesis process, difficulty in solvent recovery, influence on flame retardancy and environmental pollution in the existing production process. The invention provides an aromatic and aliphatic chain co-brominated high-molecular polymer, which sequentially comprises the following steps: preparing a brominated quaternary ammonium salt solution from liquid bromine and quaternary ammonium salt, and carrying out bromination reaction on the brominated quaternary ammonium salt solution and a styrene-butadiene polymer to obtain an aliphaticchain bromide; carrying out bromination reaction on the aliphatic chain bromide and a brominating agent under the action of a catalyst to obtain an aromatic bromination product; and carrying out post-treatment to obtain the aromatic and aliphatic chain co-brominated high-molecular polymer. The polymer and preparation method are widely applied to the technical field of flame retardant synthesis.
Owner:SHANDONG RUNKE CHEM
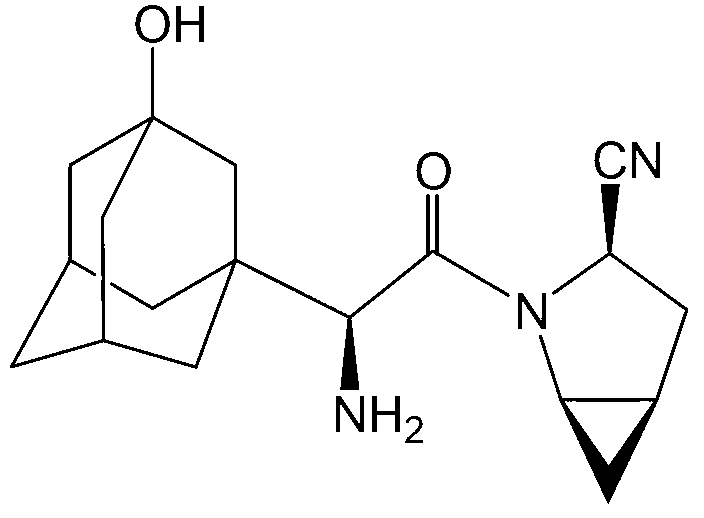
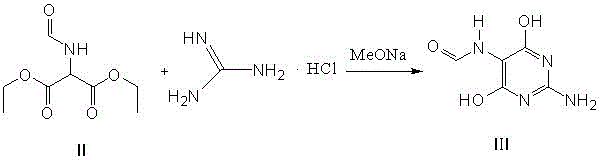
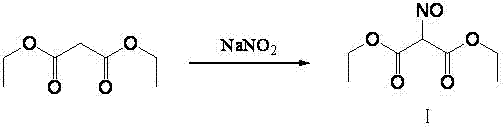
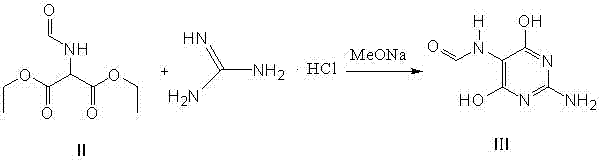
Method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine
The invention relates to a method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine. The method comprises the following steps of: performing nitrosation on malonic acid diethyl ester and acetic acid serving as raw materials and sodium nitrite at first; then, performing reduction and formylation with formic acid in the presence of zinc powder to form formyl amino malonic acid diethyl ester; finally, performing condensation and cyclization with guanidine hydrochloride to produce 2-amino-4,6-dichloro-5-formamido pyrimidine; performing chlorination by using quaternary ammonium salt as a catalyst; fractionally performing hydrolysis under an alkali action to obtain a product. The method has easily-available raw materials, and is short in reaction time, simple in aftertreatment and high in hydrolysis selectivity; the cost is obviously reduced; the total yield is up to 74 percent and the purity of the product is up to 99.0 percent.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole
ActiveCN101074215AGood removal effectMild reaction conditionsOrganic active ingredientsOrganic chemistryCyanideToluene
Production of 1-(3,5-2(2,2-dimethyl) methyl cyanide) benzyl pyrrodiazole is carried out by taking 3,5-2(2,2-dimethyl) ethyl-methyl) toluene (II) as raw material, synthesizing to obtain anatritra intermediate 3,5-2(2,2-dimethyl) ethyl-methyl) toluene bromide (III) and reacting with 1,2,4-triazanezole to obtain final product. It's simple and convenient, has less impurities and better product quality.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Bicyclol preparation method and intermediate compound thereof
ActiveCN106243079AMild reaction conditionsEasy to operateOrganic chemistryMethylenedioxyHydroxymethyl
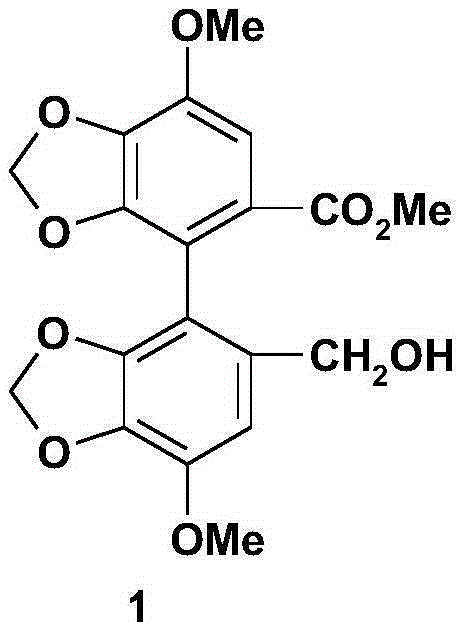
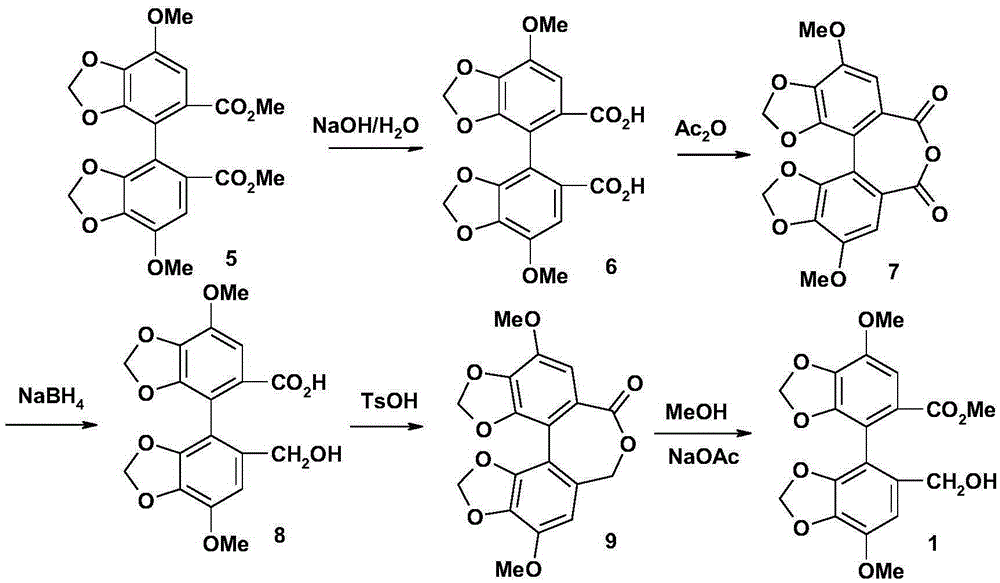
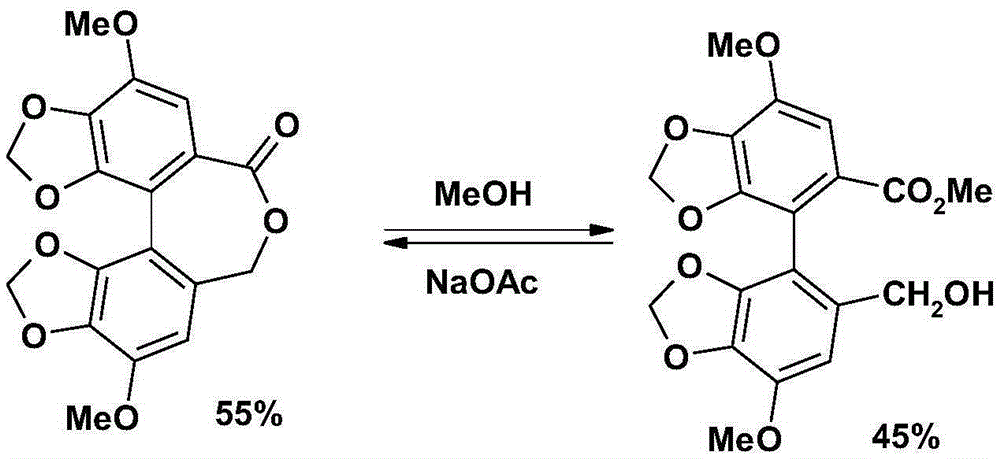
The invention relates to a bicyclol preparation method and an intermediate compound thereof. Concretely speaking, the invention relates to the preparation method of bicyclol which is 4,4'-dimethoxy-5,6,5',6'-bis(methy-lenedioxy)-2-hydroxymethyl-2'-methoxycarbonyl-biphenyl shown in a formula (1), and the intermediate compound thereof.
Owner:ZHEJIANG AUSUN PHARMA
Pleuromutilin compound, preparation method of pleuromutilin compound, polymorphism and preparation method of polymorphism
InactiveCN105399684AThe synthesis process is simpleThe post-processing method is simpleAntibacterial agentsOrganic chemistry methodsEscherichia coliThio-
The invention discloses a preparation method of a pleuromutilin compound, a polymorphism and a preparation method of the polymorphism. The pleuromutilin compound comprises a 14-O-[(4-amino-6-hydroxy-pyrimidine-2-yl) thio acetyl] matrix shown in a structural formula (i) and / or a 14-O-[(4-amino-6-one-pyrimidine-2-yl) thio acetyl] matrix shown in a structural formula (ii). The novel pleuromutilin compound disclosed by the invention has obvious inhibiting effects on multiple drug resistant bacteria such as methicillin resistant staphylococcus aureus and staphylococcus epidermidis as well as common pathogenic bacteria such as escherichia coli and streptococcus mastitidis in veterinary clinics, so that the pleuromutilin compound can be applied to preparation of antibacterial drugs and particularly anti-drug resistant bacterium drugs.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Preparation method of chiral pantoprazole and sodium salt thereof
The invention provides a preparation method of chiral 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)-methyl]-sulfinyl]-1H-benzimidazole (pantoprazole) and a sodium salt thereof. The method comprises the following steps: in an organic solvent, adding an organic alkali in the presence of a chiral titanium complex prepared from a diamide R,R or S,S-tartrate ligand, a metal titanium reagent and water, and oxidizing prochiral thioether of pantoprazole by a hydrogen peroxide oxidizer to obtain the corresponding pantoprazole containing rich single antipode. The method has the advantages of high enantioselectivity, mild reaction, simple after-treatment process, fewer procedure steps, low solvent consumption and low cost, and is very simple to operate.
Owner:CHANGZHOU INST OF CHEM
Method for producing liquid fuel by using biomass
ActiveCN110467940AImprove combustion qualityFully convertedLiquid hydrocarbon mixture productionHydrocarbon oils treatmentFiberLiquid fuel
The invention provides a method for producing a liquid fuel by using biomass. The liquid fuel is obtained by performing pretreatment, a pyrolytic reaction and a catalytic cracking reaction on the biomass. The biomass is composed of pine needles, bamboo wood, tung tree branches, ginger stems and leaves, Chinese toon branches, sweet potato seedlings, Enteromorpha prolifera, leftovers from a slaughterhouse and the like, wherein the pine needles, the bamboo wood, the tung tree branches, the ginger stems and leaves, the Chinese toon branches and the sweet potato seedlings contain volatile oil, grease, cellulose, lignin and the like; Enteromorpha prolifera contains a large amount of carbohydrates, proteins, crude fibers, fatty acids and the like; and the leftovers from the slaughterhouse are rich in grease. The above biomass raw materials are subjected to the pyrolysis reaction and the catalytic cracking reaction for sufficient conversion, and the obtained liquid fuel is excellent in combustion quality.
Owner:台州镘霓电子商务有限公司
Synthesis method of triaminoguanidinium nitrate
The invention relates to a synthesis method of triaminoguanidinium nitrate. The defects of foreign literature report technological approaches are overcome, low-price industrial-grade guanidine nitrate and hydrazine hydrate are used as raw materials, deionized water is used as solvent, nitric acid is used as a catalyst, and triaminoguanidinium nitrate is synthesized through a two-step reaction method. By means of the method, the synthesis time is short, raw materials are low in price, can be easily obtained and are high in utilization rate, the synthesis route is safe, the post-processing method is simple, the synthesized target compound product is high in yield and purity, and byproducts can be recycled. Triaminoguanidinium nitrate synthesized through the method is mainly used for clean type gas generator charging, missiler roll control, start of turbopumps used for servo mechanisms, emergent starters of jet aircrafts, automobile air bags and the like.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
Preparation of 5'-deoxynucleoside monophosphate
ActiveCN101503432AAdaptableReaction is easy to controlSugar derivativesSugar derivatives preparationOrganic solventPhosphoric acid
The invention discloses a preparing method of 5'-deoxyribonucleoside monophosphate, which comprises the following steps: (1) mixing 2'-deoxyribonucleoside, phosphorus oxychloride and trialkyphosphate to obtain a crude solution containing 5'-deoxynucleotide; (2) hydrolyzing the 5'-deoxynucleotide crude solution obtained from the step (1) in a solution with pH higher than or equal to 8.0 so as to an obtain hydrolysis liquid; (3) mixing an organic solvent, i.e. halogenated hydrocarbon, with the hydrolysis liquid obtained from the step (2), and extracting the mixture to obtain an organic phase and an aqueous phase; (4) condensing the aqueous phase to obtain a condensed liquid; and (5) placing the condensed liquid obtained from the step(4) at a temperature of 10-15 DEG C to obtain the solid 5'-deoxyribonucleoside monophosphate.
Owner:SHANGHAI ZHAOWEI TECH DEV
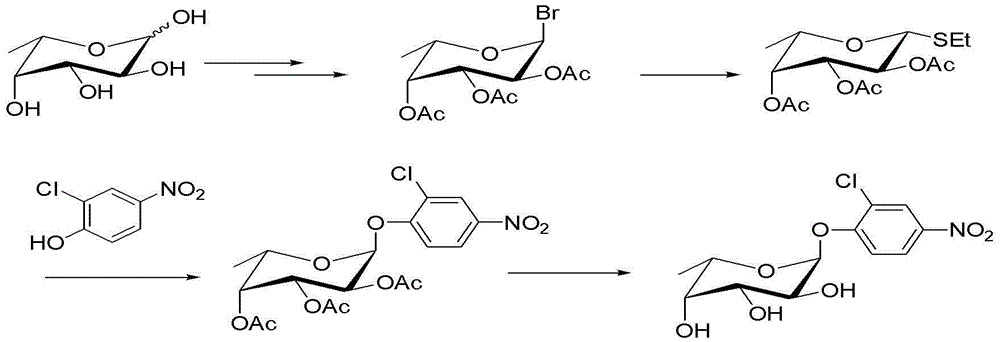
Method for preparing 2-chloro-4-nitrophenyl-alpha-L-fucoside
InactiveCN103059076ALow priceThe post-processing method is simpleSugar derivativesSugar derivatives preparation2-chloro-4-nitrophenolBromine
The invention relates to a method for preparing 2-chloro-4-nitrophenyl-alpha-L-fucoside and belongs to the field of medicine production. The method comprises the following steps of: performing hydroxyl protection and bromination on L-fucoside to obtain 1-bromo-2,3,4-triacetyl-alpha-L-fucoside; reacting the 1-bromo-2,3,4-triacetyl-L-fucoside with ethanethiol to obtain ethyl-1-mercapto-2,3,4-triacetyl-beta-L-fucoside; reacting the ethyl-1-mercapto-2,3,4-triacetyl-beta-L-fucoside with 2-chloro-4-nitrophenol to obtain 2-chloro-4-nitrophenyl-2,3,4-triacetyl-alpha-L-fucoside; and removing protection groups from the 2-chloro-4-nitrophenyl-2,3,4-triacetyl-alpha-L-fucoside to obtain the 2-chloro-4-nitrophenyl-alpha-L-fucoside. According to a product obtained by the method, alpha-configuration products are high in proportion; and the method has a good industrial production prospect.
Owner:BEIJING LEADMAN BIOCHEM
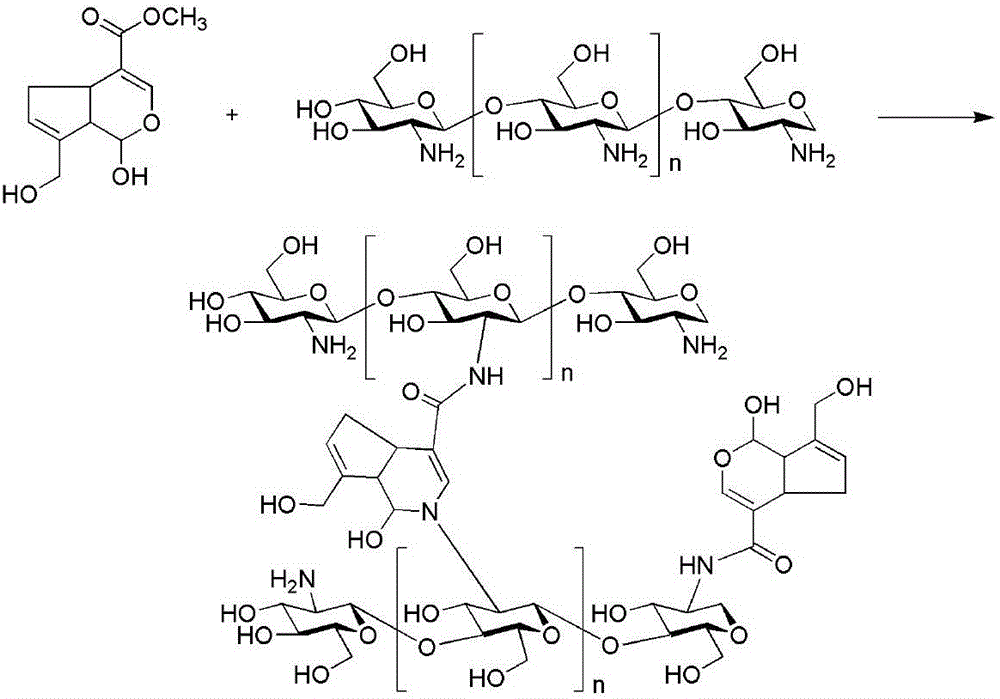
Preparation method of bio-functionalized chitosan hydrogel
InactiveCN105920675AThe post-processing method is simpleImprove efficiencyTissue regenerationProsthesisGenipinCytotoxicity
The invention belongs to the field of tissue engineering biological materials, and specifically relates to a preparation method of bio-functionalized chitosan hydrogel. The preparation method comprises the following steps: preparing genipin solutions with different weight percentages: (1) individually dissolving 0.005 gram of genipin, 0.01 gram of genipin, and 0.02 gram of genipin into 1 mL of alcohol (75%) to prepare a genipin solution (0.5%), a genipin solution (1.0 %), and a genipin solution (2.0%); (2) preparing a chitosan solution: dissolving 1.5 grams of chitosan into 100 mL of acetic acid solution (3%) to prepare a chitosan solution (1.5%), adding biological molecules containing amino groups, and stirring the mixture in a bottle for a whole night; (3) mixing two solutions according to different ratios, and allowing the mixed solution to stand still for 24 to 72 hours to obtain the functionalized chitosan hydrogel; wherein the mole ratio of genipin to biological molecules containing amino groups is maintained in a range of 10:5 to 10:1; and the mole ratio of chitosan to genipin is in a range of 300:1 to 30:10. The operation is simple, the cost is low, the reaction conditions are mild and matured, the cytotoxicity is little or none, and the method is universal.
Owner:SUZHOU UNIV
Process for preparing alkylphenol formaldehyde oligomer sulfonate
InactiveCN102584641AModerate degree of polymerizationEasy to operateSulfonic acid preparationSulfonateAqueous sodium hydroxide
The invention discloses a process for preparing alkylphenol formaldehyde oligomer sulfonate. The process comprises the following steps of: adding alkylphenol and a catalyst into a reaction kettle, heating with stirring, dripping a formaldehyde solution or paraformaldehyde, continuously stirring, and raising temperature to reaction temperature, adding residual formaldehyde solution or paraformaldehyde, continuously reacting, and after reaction is finished, adding a sodium hydroxide aqueous solution to adjust pH of a reaction product to be 6.5 to 8.0 to obtain alkylphenol formaldehyde oligomer; and adding the alkylphenol formaldehyde oligomer into the reaction kettle, adding a solvent to dissolve, opening a stirrer, stirring and heating to 30 to 80 DEG C, adding a sulfonating agent, continuously reacting for 30 to 90 minutes, neutralizing a sulfonating product by using alkali until pH is 7 to 8, and evaporating to remove the solvent to obtain the alkylphenol formaldehyde oligomer sulfonate. The process has the advantages that alkylphenol and formaldehyde are used as raw materials, the process is simple, and industrialization is easy to realize.
Owner:CHINA RES INST OF DAILY CHEM IND
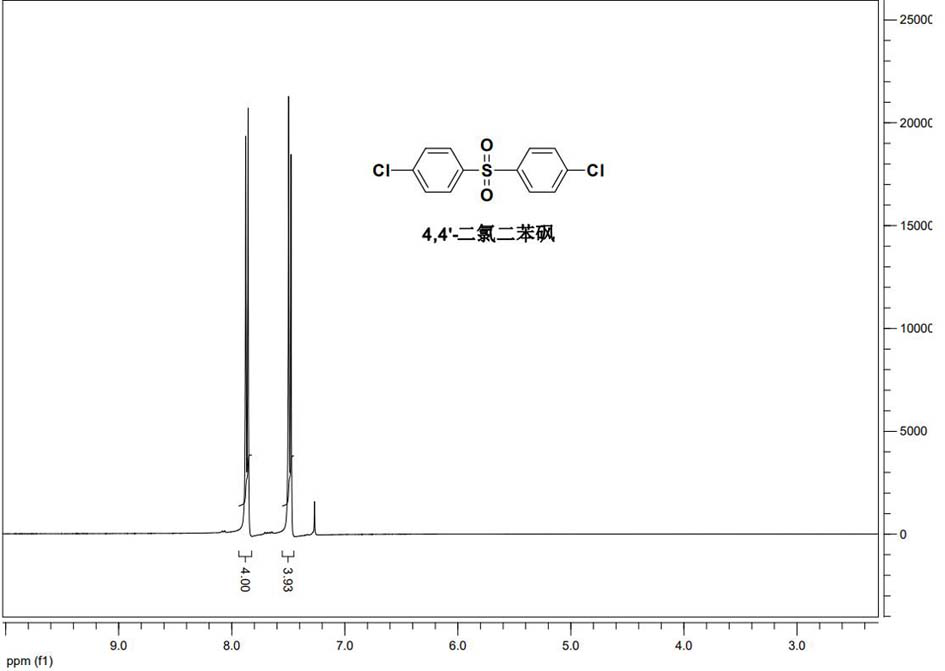
Preparation method of 4, 4 '-dichlorodiphenyl sulfone
ActiveCN113387851ALow priceHigh catalytic efficiencyOrganic chemistryOrganic compound preparationPtru catalystMolybdate
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of high-purity 4, 4-dichlorodiphenyl sulfone. The preparation method of the high-purity 4, 4-dichlorodiphenyl sulfone comprises the following steps: firstly, mixing molybdate, organic dicarboxylic acid and hydrogen peroxide, dropwise adding the mixture into a mixed solution of 4, 4-dichlorodiphenyl sulfoxide and a solvent for reaction, and after the reaction is completed, performing post-treatment to obtain the high-purity 4, 4-dichlorodiphenyl sulfone and residual filtrate; the residual filtrate is directly used for the next round of reaction after being layered; the molar ratio of the 4, 4-dichlorodiphenyl sulfoxide to the molybdate to the organic dicarboxylic acid to the hydrogen peroxide is 1: (0.01-0.05): (0.02-0.1): (1-3). The invention provides the preparation method of the high-purity 4, 4-dichlorodiphenyl sulfone, the catalyst is low in cost, the preparation steps are simple, the post-treatment steps are simple, the reaction residual filtrate is circularly and efficiently utilized, the purity is high, and the yield is high.
Owner:FUHAI (DONGYING) ADVANCED MATERIAL TECH CO LTD
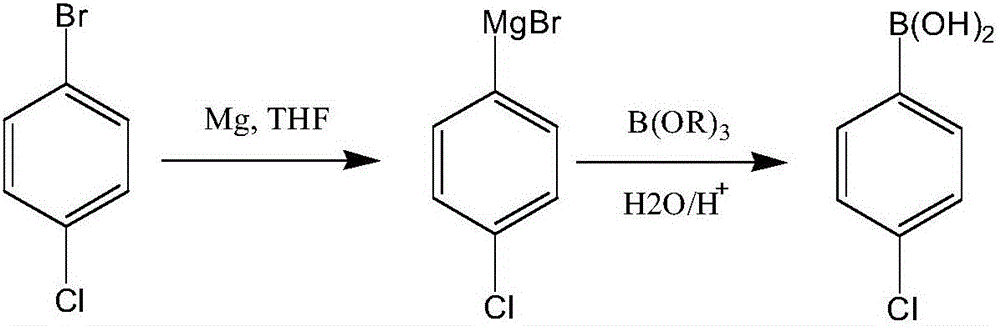
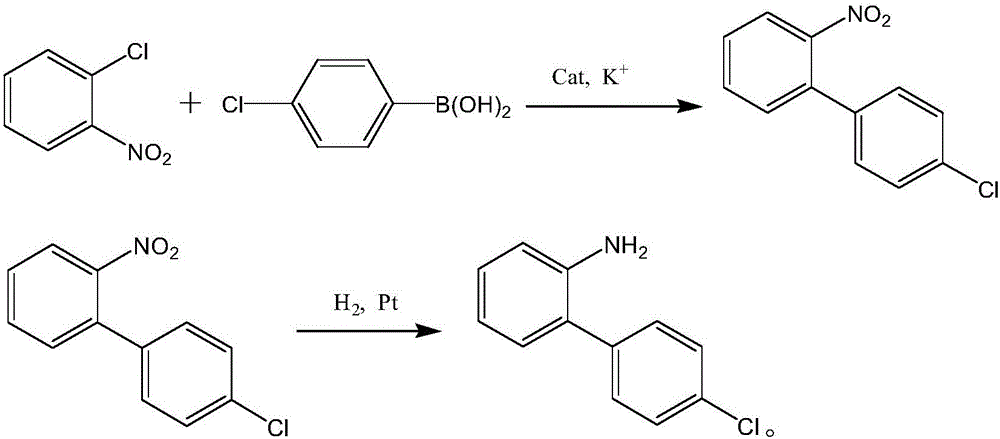
Synthesis process of boscalid intermediate 2-(4-chlorphenyl)phenylamine
InactiveCN106748804AReduce pollutionGood workmanshipOrganic compound preparationGroup 3/13 element organic compoundsSuzuki reactionCoupling
The invention discloses a synthesis process of boscalid intermediate 2-(4-chlorphenyl)phenylamine. Chlorophenylboronic acid and o-chloronitrobenzene are taken as starting raw materials, and a main reaction consisting of the two steps of Suziki and hydrogenation reduction is carried out to prepare the 2-(4-chlorphenyl)phenylamine. 1, optimal reaction steps determined through continuous testing are adopted, a proper solvent is selected preferably, and the 2-(4-chlorphenyl)phenylamine is prepared at a proper temperature and under a proper pressure, so that a superior process, high yield and high working efficiency are realized, no noble metal coupling agent is needed, the environmental pollution is lowered, raw materials are readily available, and the production cost is lowered; 2, a target product is obtained by obtaining a solid through concentration and cooling, so that a posttreatment method is simplified effectively, and meanwhile the content and high yield of the product are ensured effectively; 3, the Suzuki reaction steps are simple, filtrate left after the reaction can be directly applied to a next-step hydrogenation reduction reaction, operation is easy, no pollutant waste is produced in the hydrogenation reduction reaction, and the environment and the health of operating personnel are protected.
Owner:ZHEJIANG RONGKAI TECH DEV
Method for producing saxagliptin
The invention provides a method for producing saxagliptin. The method comprises the following steps of: 1, providing a compound d; and 2, reacting the compound d, isopropanol and concentrated hydrochloric acid at the temperature of between 50 and 80 DEG C, and collecting the product to obtain the saxagliptin. Compared with the prior art, the method has the advantages that the compound d is directly converted into the target product, so that the reaction rate is greatly improved, the reaction yield is improved, the post treatment method is simplified, and industrial wastewater is greatly reduced.
Owner:SHANGHAI TWISUN BIO PHARM
Preparation method of 2-amino-4,6-dichloro-5-carboxamidopyrimidine
The invention relates to a method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine. The method comprises the following steps of: performing nitrosation on malonic acid diethyl ester and acetic acid serving as raw materials and sodium nitrite at first; then, performing reduction and formylation with formic acid in the presence of zinc powder to form formyl amino malonic acid diethyl ester; finally, performing condensation and cyclization with guanidine hydrochloride to produce 2-amino-4,6-dichloro-5-formamido pyrimidine; performing chlorination by using quaternary ammonium salt as a catalyst; fractionally performing hydrolysis under an alkali action to obtain a product. The method has easily-available raw materials, and is short in reaction time, simple in aftertreatment and high in hydrolysis selectivity; the cost is obviously reduced; the total yield is up to 74 percent and the purity of the product is up to 99.0 percent.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
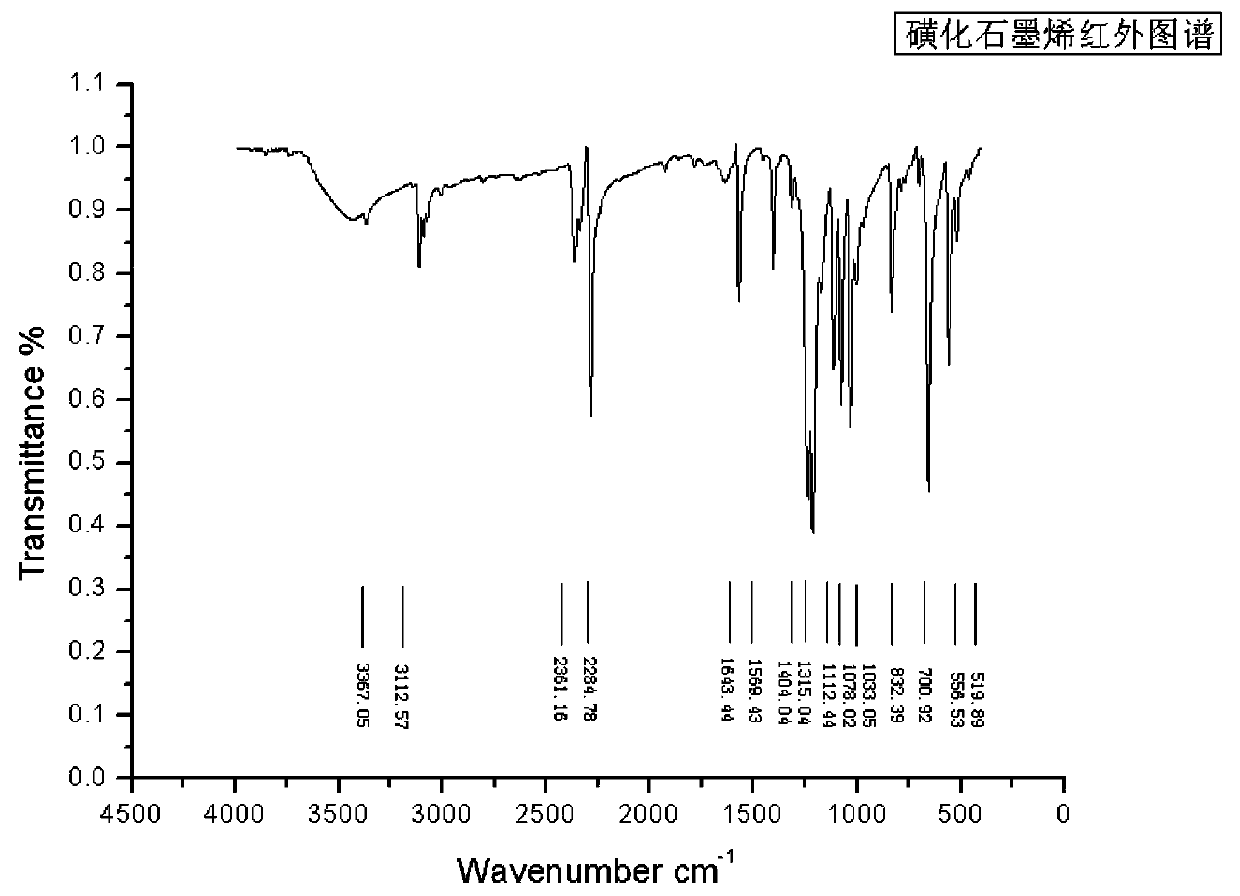
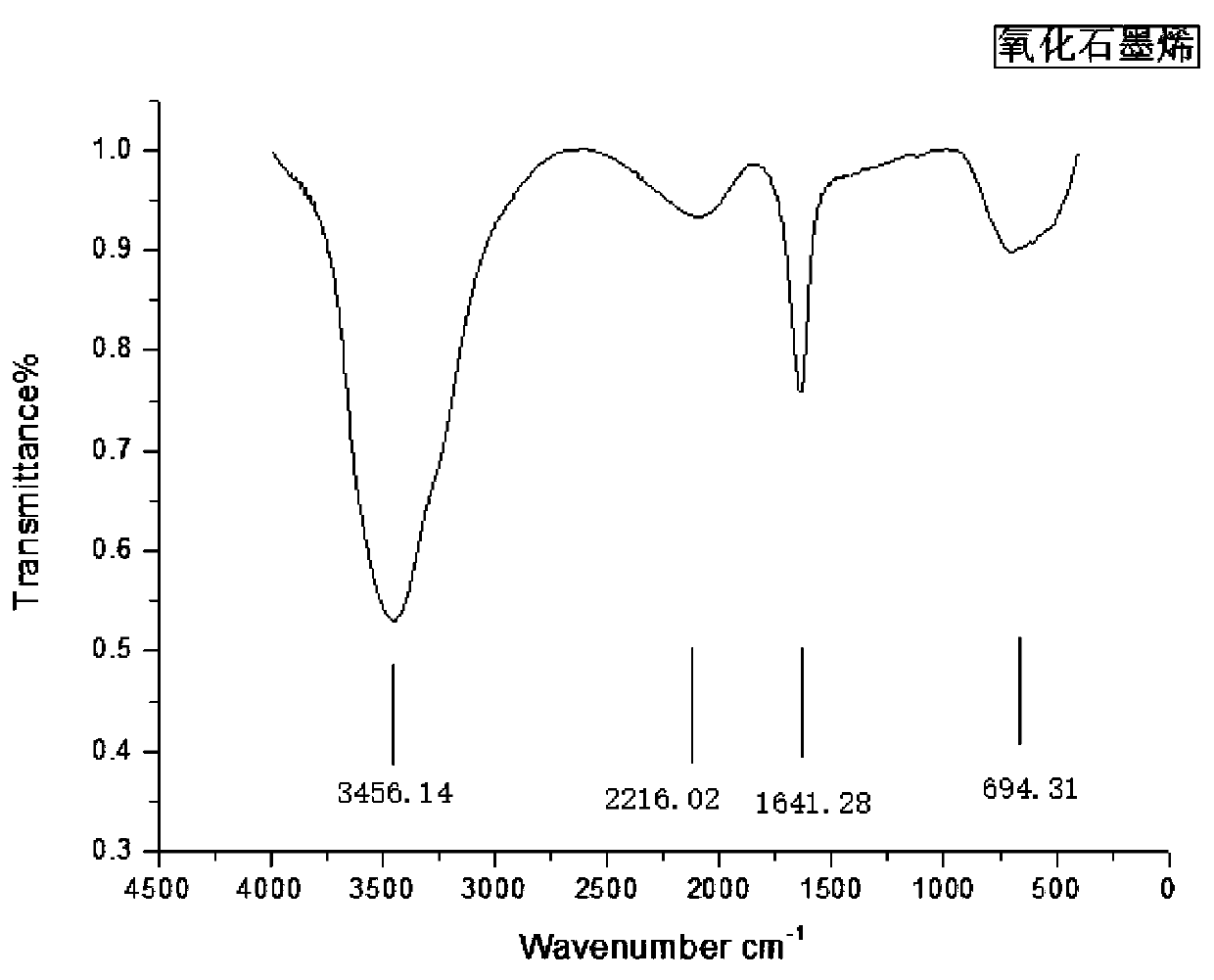
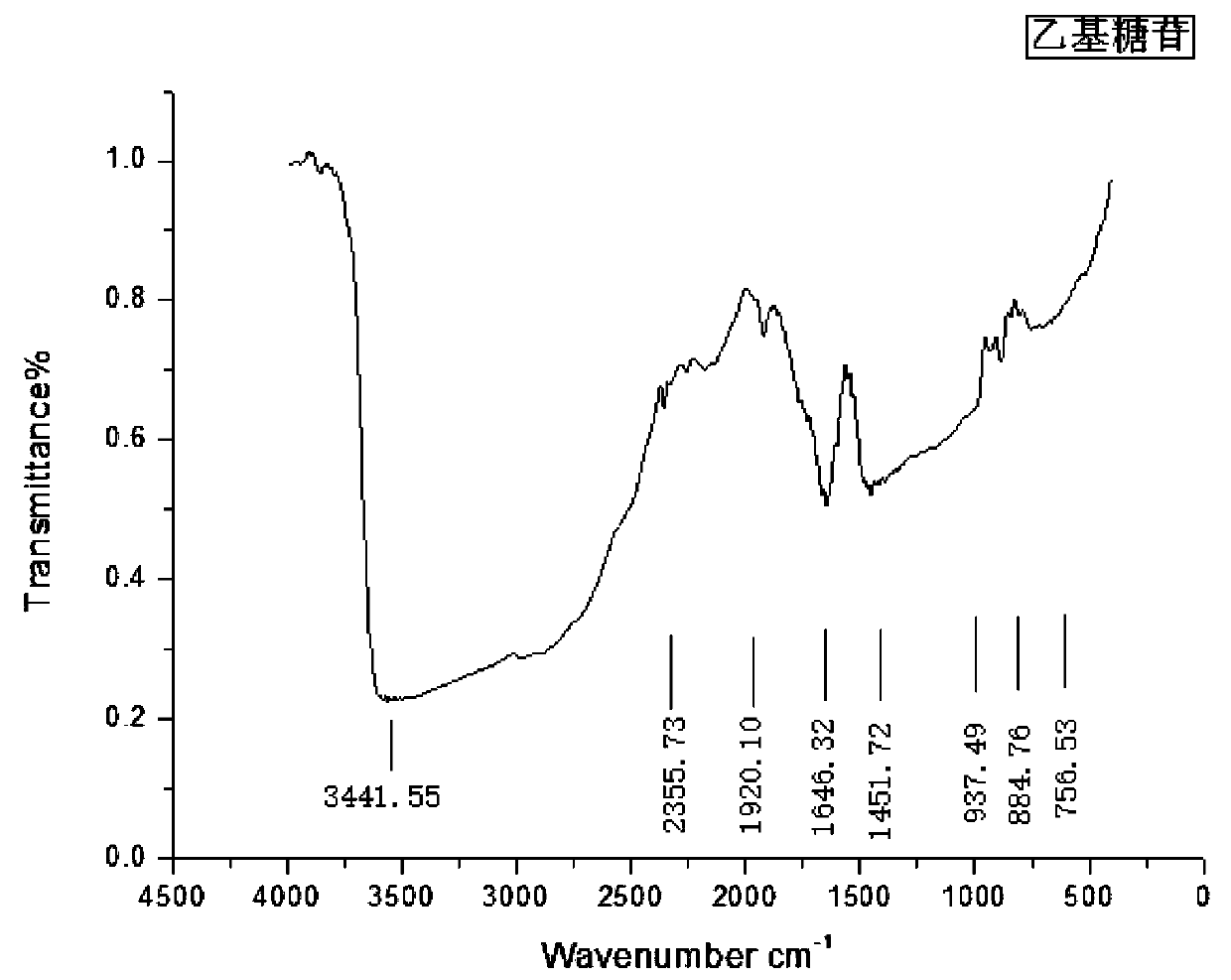
Preparation method of alkyl polyglycoside by using sulphonating graphene as catalyst
InactiveCN110684060AGood dispersionLarge specific surface areaSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsSulfanilic acidBenzene
The invention discloses a preparation method of alkyl polyglycoside by using sulphonating graphene as a catalyst, and mainly aims to solve the technical problems of incomplete transformation and highproduction cost existing in a conventional synthesis method. The preparation method comprises the following steps of performing diazotization modification on sulfanilic acid, enabling the sulfanilic acid after the diazotization modification to react with active hydroxyls and epoxy groups on the surface of oxidized graphene to obtain an oxidized graphene loaded benzene sulfonic acid catalyst, enabling glucose and fatty alcohol to be mixed and dissolve, and by using the sulphonating graphene as the catalyst, performing one-step glycosidation so as to obtain the alkyl polyglycoside.
Owner:上海吉奉生物科技有限公司 +1
Preparation method of disperse dyestuff
ActiveCN103232409ALow toxicitySimple processMonoazo dyesOrganic compound preparationDisperse dyeHydrogen
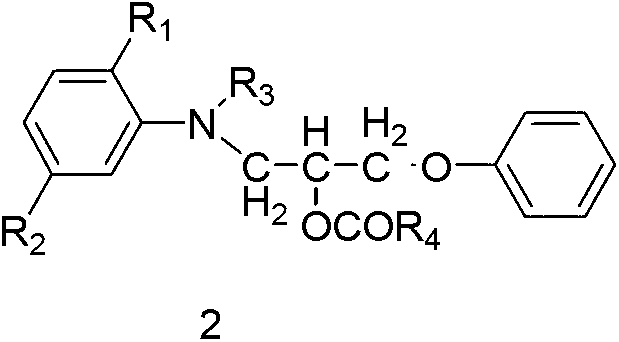
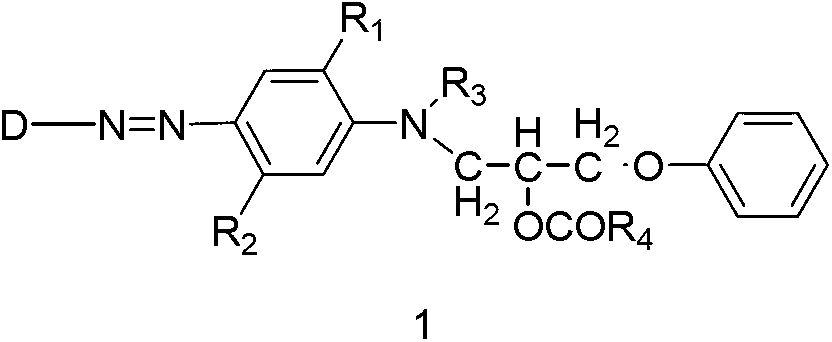
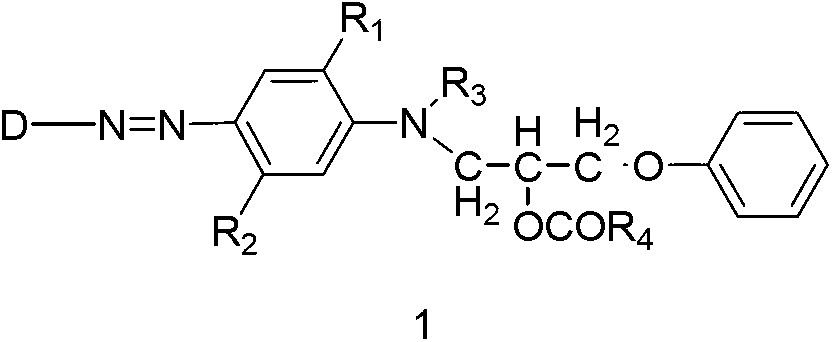
The invention discloses a preparation method of a disperse dyestuff, and provides a preparation of a compound represented by the formula 1. The method comprises the steps that: in a solvent and under an acid catalyzing condition, diazonium salt represented by s formula 3 is subjected to a coupling reaction with a compound represented by a formula 2, such that the compound 1 is obtained, wherein A is Cl<->, CH2COO<->, H2PO4<->, HSO4<->, or NO3<->; R1 is C1-4 alkoxy or hydrogen; R2 is C1-4 acylamino or hydrogen; R3 is C1-4 alkyl; and R4 is C1-4 alkyl; and D is represented as the following. The disperse dyestuff preparation method provided by the invention has the advantages of simple process, easy operation, simple post-treatment method, low raw material toxicity, and environment-friendliness.
Owner:SHANGHAI ANOKY GRP
Method for preparing tetrabenzyl pyran type hexose
ActiveCN104031101AEasy to operateModerate acidityPhysical/chemical process catalystsSugar derivativesHydrogen SulfatePtru catalyst
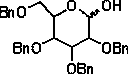
The invention provides a method for preparing tetrabenzyl pyran type hexose, belonging to the field of sugar compounds. The method adopts a supported solid-acid catalyst namely silica gel supported sulfuric acid-hydrofluoric acid, performs catalytic hydrolysis on tetrabenzyl pyran type hexose glucoside, is simple in operation, is environment-friendly and practical, and simplifies an after-treatment method. The method comprises the steps of adding tetrabenzyl pyran type hexose glucoside and an organic solvent at room temperature, adding silica gel supported sulfuric acid-hydrofluoric acid, completing reaction, and then performing after-treatment to obtain tetrabenzyl pyran type hexose. The preparation method provided by the invention is simple in operation and easy in reaction control, reduces the cost, and is beneficial for environmental protection.
Owner:济南尚博医药股份有限公司
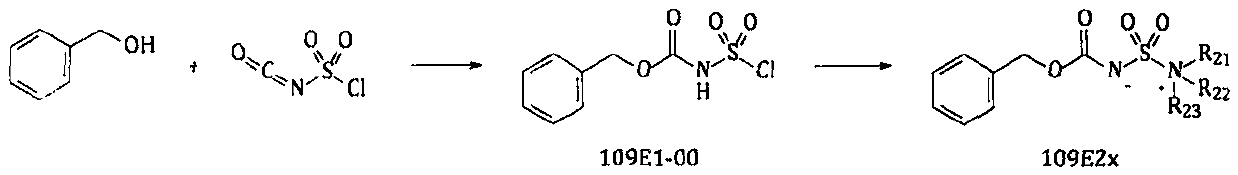
Preparation method of eluxadoline intermediate compound
ActiveCN106866463AEase of industrial applicationLow priceCarbamic acid derivatives preparationOrganic compound preparationPropanoic acidTert-Butyloxycarbonyl protecting group
The invention provides a preparation method of an eluxadoline intermediate compound, and concretely discloses a method for preparing (S)-2-tertbutyloxycarbonyl amino-3-(4-carbamyl-2,6-dimethyl phenyl) propanoic acid shown by a formula 11. The method comprises the following steps of Step I, obtaining a compound shown by a formula 4 through a compound shown by a formula 1; Step II, obtaining a compound shown by a formula 5 by the compound shown by the formula 4; Step III, obtaining a compound shown by a formula 6 by the compound shown by the formula 5; Step IV, protecting amino groups in the compound shown by the formula 6 to obtain a compound shown by a formula 9; Step V, performing selective hydrolysis on the compound shown by the formula 9 to obtain a compound shown by a formula 10; then, performing ammonolysis to obtain the compound shown by the formula 11. The process routes of the method use the fire-new design; intermediate compounds obtained by the reaction in each step are all synthesized for the first time; a method of firstly performing carbonylation and then performing asymmetrical reduction is used in the process routes; the asymmetrical reduction is realized in the later stage; the catalyst consumption is favorably reduced; the cost is reduced. The formulas are shown as the accompanying drawing.
Owner:富乐马鸿凯(大连)医药有限公司
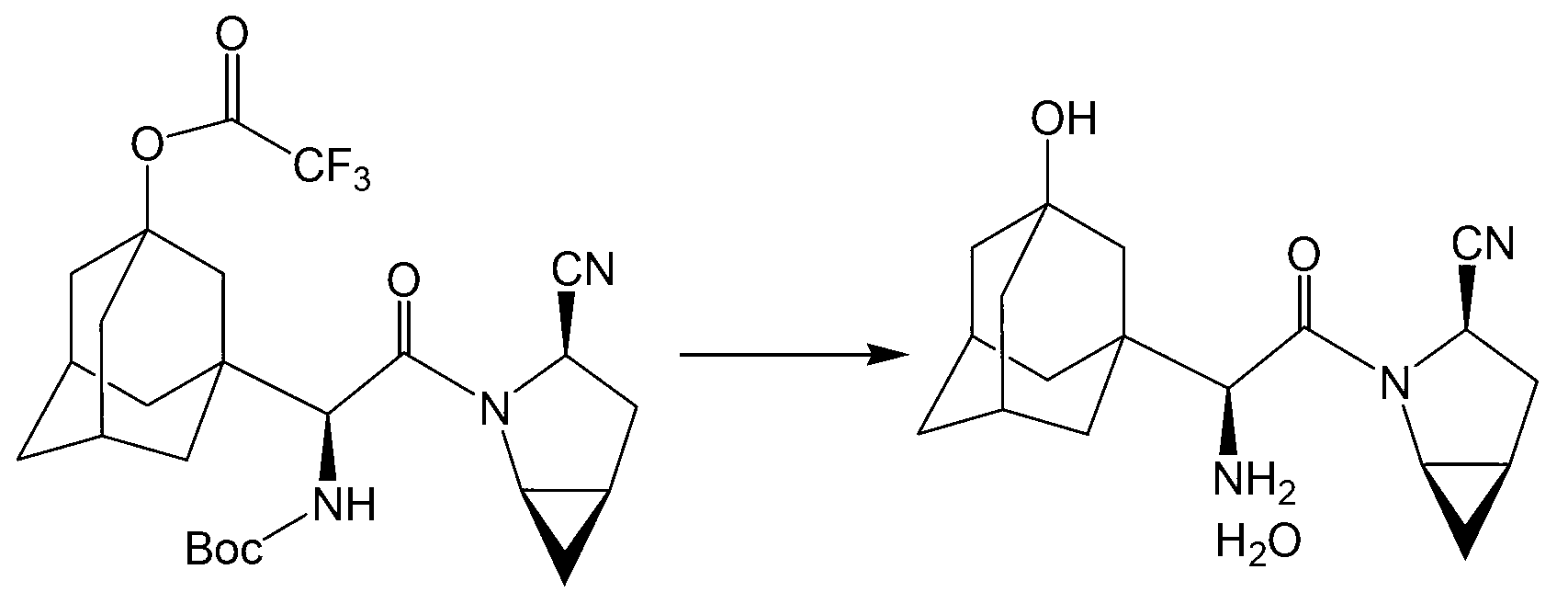
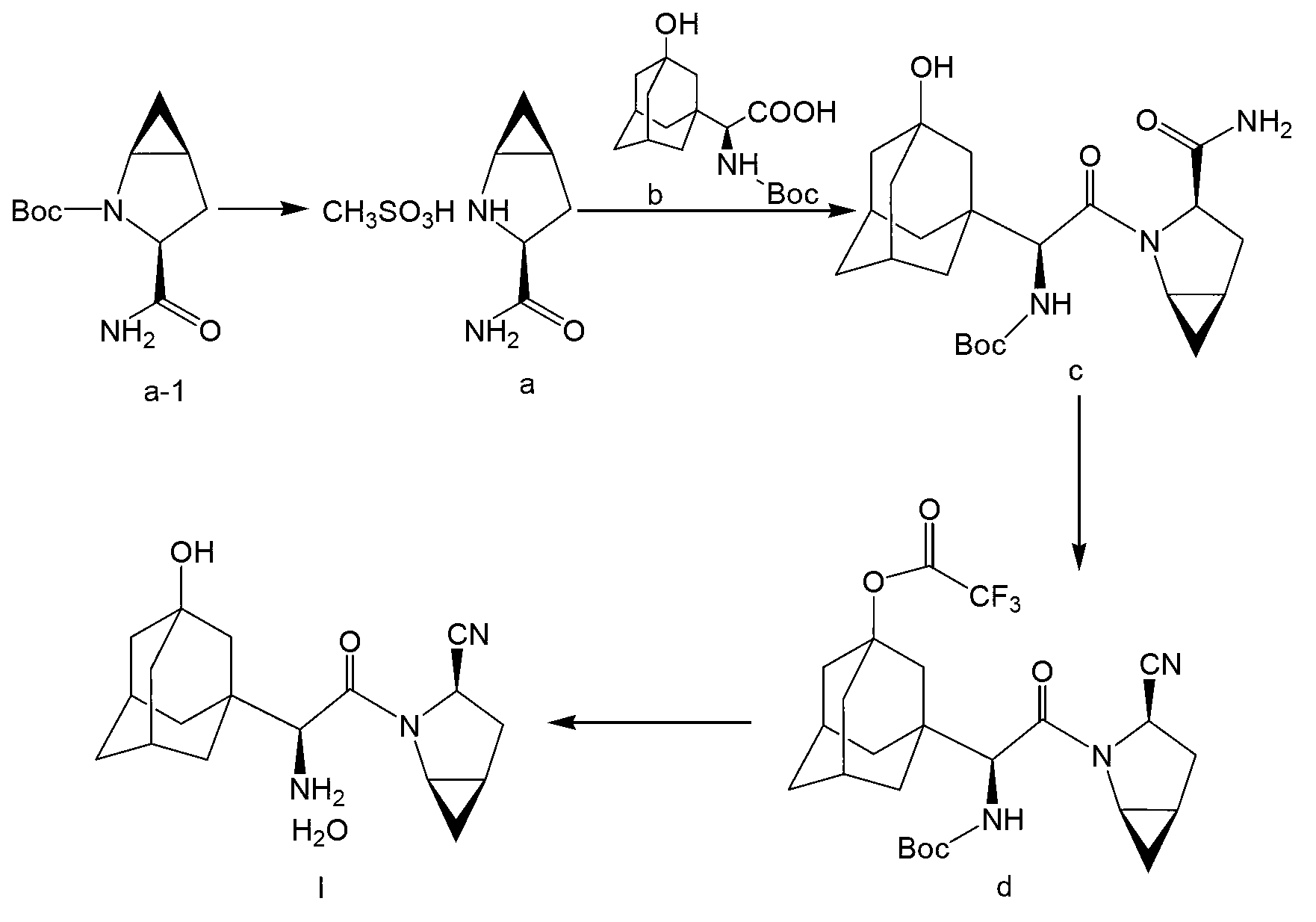
A kind of preparation method of prothioconazole intermediate
ActiveCN107445909BThe post-processing method is simpleShort reaction timeOrganic chemistryChlorobenzeneThioketone
The invention relates to a preparation method of a prothioconazole intermediate. The preparation method comprises the following steps: dissolving [1-(2-chlorophenyl)-2-(1-chlorocyclopropyl)-2-hydroxy]-propyl hydrazine into methyl benzene; dropwise adding a formaldehyde aqueous solution or a polyformaldehyde aqueous solution under 30 to 35 DEG C; then adding in thiocyanate after completion of dropwise adding; then dropwise adding hydrochloric acid or sulfuric acid aqueous solution; keeping warm and reacting for 50 to 70 minutes after completion of dropwise adding; directly using a reaction solution for follow-up reaction or filtering the reaction solution after cooling; thus obtaining the prothioconazole intermediate 2-(1-chloro-cyclopropyl-1-yl)-1-(2-chlorophenyl)-2-hydroxy-3-(1,2,4-triazolidine-5-thioketone-1-yl)-propane. Through optimization on a whole route, for example, reaction temperature, reagents, a feeding procedure and the like, of the preparation method, an after-treatment method is simple, three wastes such as waste gas, waste water and waste residues are few, the reaction time is shortened, the purity and the yield of a product are higher, and the preparation method is suitable for industrial production.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Anionic surfactant having bactericidal activity and preparation method thereof
InactiveCN103949185AImprove stabilityReasonable designOrganic chemistryTransportation and packagingSulfamic acidSURFACTANT BLEND
The invention discloses an anionic surfactant having bactericidal activity and a preparation method thereof. The anionic surfactant has a structure shown in the following general formula. Cyanuric chloride, alicyclic amine, sulfamic acid and aminobenzimidazole as raw materials are mixed according to a reasonable feeding ratio and undergo a three-step synthesis reaction to produce the anionic surfactant. The anionic surfactant has surfactant properties and has bactericidal activity. The anionic surfactant having bactericidal activity solves the problem of poor compatibility of a surfactant and a bactericide, overcomes charge limit, widens an application range of the anionic surfactant having the bactericidal activity, can be treated easily in a use later period, has a less use amount and has important theoretical and practical significances. In the general formula, R1 represents -(CH2)5CH3, -(CH2)7CH3, -(CH2)9CH3 or -(CH2)11CH3 and R2 represents -CH2-, -(CH2)2-, -(CH2)3- or -C6H4.
Owner:ZHONGBEI UNIV
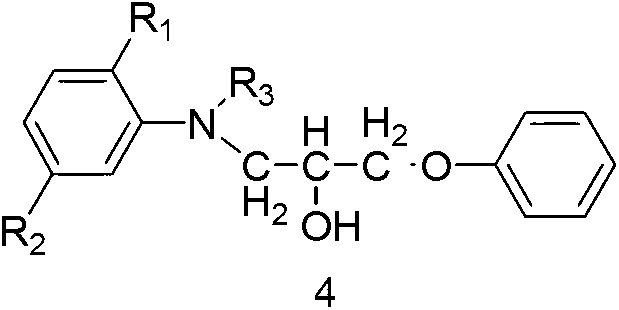
Easter compounds and preparation method thereof
ActiveCN103232723ALow toxicitySimple processMonoazo dyesOrganic compound preparationHydrogenAlkoxy group
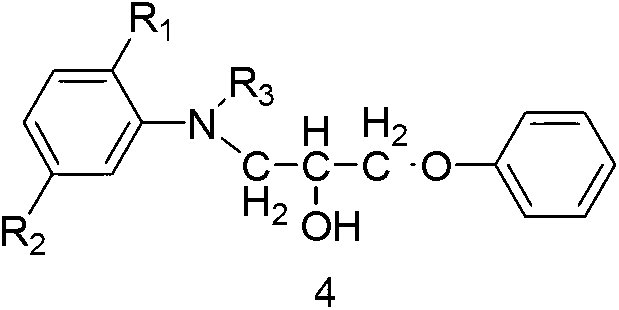
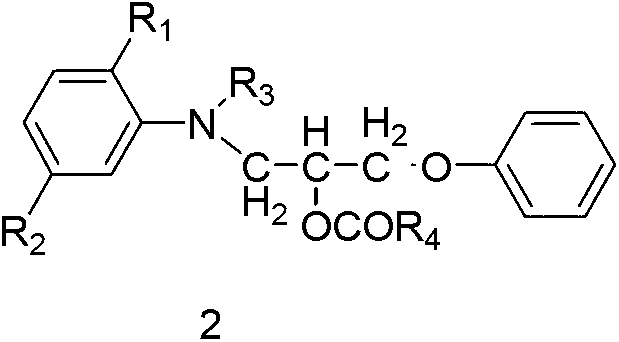
The invention discloses ester compounds and a preparation method thereof. The invention provides the ester compounds represented by a formula 2, wherein R1 is C1-4 alkoxy or hydrogen, R2 is C1-4 acylamino or hydrogen, R3 is C1-4 alkyl, and R4 is C1-4 alkyl. The preparation method of the ester compounds represented by the formula 2 has the step that: a compound 4 and anhydride represented by a formula 5 are subjected to an acylation reaction, such that the eater compound 2 is obtained.
Owner:DONGYING ANNUOQI TEXTILE MATERIALS
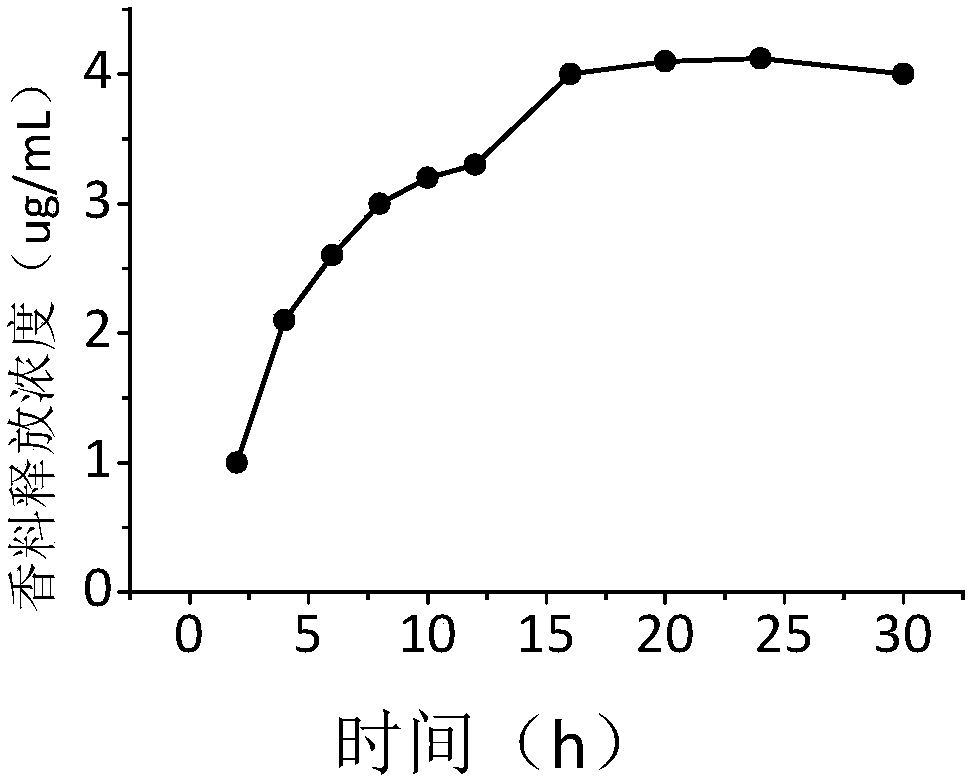
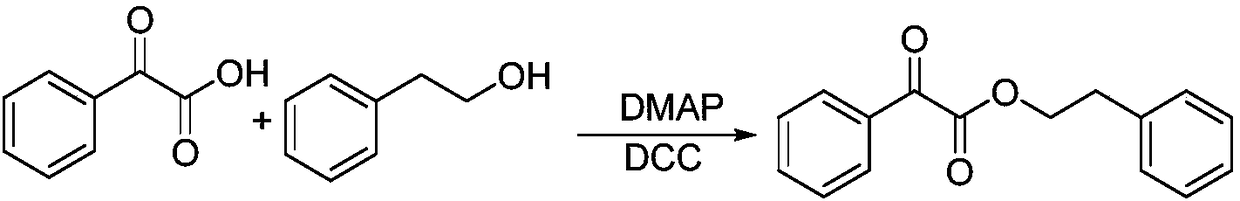
Nano spice precursor based on 2-carbonyl acetate and application thereof
ActiveCN108264642AExperiment operation is simpleEasy to industrializeEssential-oils/perfumesAryleneChemistry
The invention discloses a nano spice precursor based on 2-carbonyl acetate. The nano spice precursor based on 2-carbonyl acetate is shown as general formula (I) in the specification, wherein R is selected from H, alkyl, alkoxy, alkenyl, aryl, arylene and cycloalkyl; and R1 is selected from alkyl, alkoxy, alkenyl-containing alkyl, aryl, arylene, and alkenyl; and n is a positive of 1-20. The nano spice precursor based on 2-carbonyl acetate provided by the invention has the characteristics of simple experimental operation, easy industrialization and low cost, and can be used as a sustained release spice.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

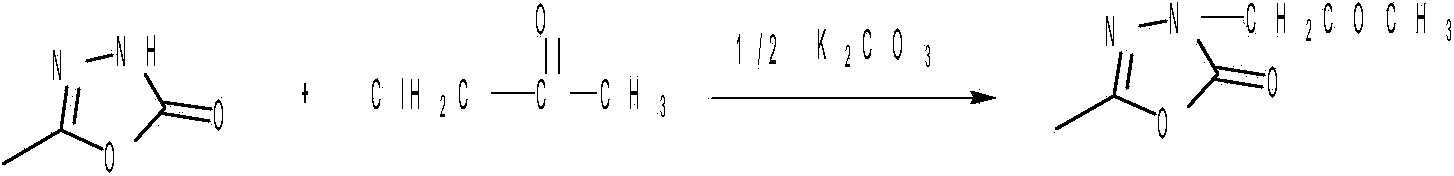
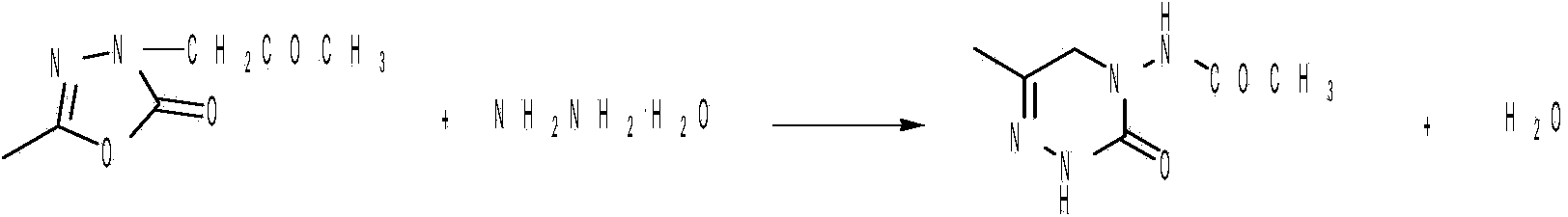
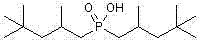
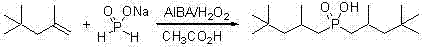
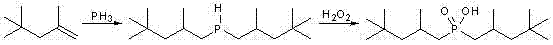
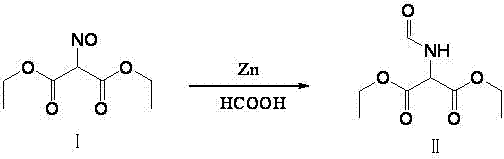
![Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole](https://images-eureka.patsnap.com/patent_img/fca283a6-d351-45ac-8f36-edd4db17f7b7/A20061002661500111.PNG)
![Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole](https://images-eureka.patsnap.com/patent_img/fca283a6-d351-45ac-8f36-edd4db17f7b7/A20061002661500121.PNG)
![Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole Production of 1-[3,5-2(2,2-dimethyl) ethylcyano] benzyl triazole](https://images-eureka.patsnap.com/patent_img/fca283a6-d351-45ac-8f36-edd4db17f7b7/A20061002661500131.PNG)