Preparation method of L-alanyl-L-glutamine
A technology of glutamine and alanyl, applied in the field of medicine, can solve the problems that hydrazine residues are difficult to completely remove, unfavorable for operation safety, and unfavorable for environmental protection, so as to reduce the steps of water extraction products, reduce production costs and The effect of three wastes and simplified post-treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 7000 mL of anhydrous methanol into a 10L reaction flask, stir, and then add 694 g of N-phthaloyl-L-alanyl-L-glutamine (the molar ratio of N-phthaloyl-L-propion Aminoacyl-L-glutamine: methylamine: = 1:6), cooled in an ice bath at 5°C~10°C, added 862g of 40% methylamine aqueous solution dropwise until the solution was clear, removed the ice bath, and slowly added the rest of the formaldehyde After the addition of the amine solution, react at room temperature for 6 hours, concentrate under reduced pressure in a water bath at 30°C to remove excess methylamine to pH = 6~7, stand for crystallization for 10 hours, filter, and dry the filter cake under normal pressure to obtain 425g of white solid, with a yield of 96%. HPLC: 99%.
[0026] Weigh 400g of the crude product, add 800ml of water to stir the solution to clarify, slowly add 4000ml of methanol dropwise, after the addition is complete, stir at room temperature for 2h, filter, and dry the filter cake at 60°C to obtain...
Embodiment 2
[0028] Add 5000 mL of absolute ethanol into a 10L reaction flask, stir, and then add 694 g of N-phthaloyl-L-alanyl-L-glutamine (the molar ratio of N-phthaloyl-L-propion Aminoacyl-L-glutamine: methylamine: = 1:4), cooling in an ice bath at 5°C~10°C, add dropwise 575g of 33% methylamine methanol solution until the solution is clear, remove the ice bath, and slowly add the rest Methylamine solution, reacted at room temperature for 8 hours after adding, concentrated under reduced pressure in a water bath at 25°C to remove excess methylamine to PH=6~7, stood for crystallization for 10 hours, filtered, and dried the filter cake under normal pressure to obtain 426g of white solid, yield 96.4% , HPLC: 99%.
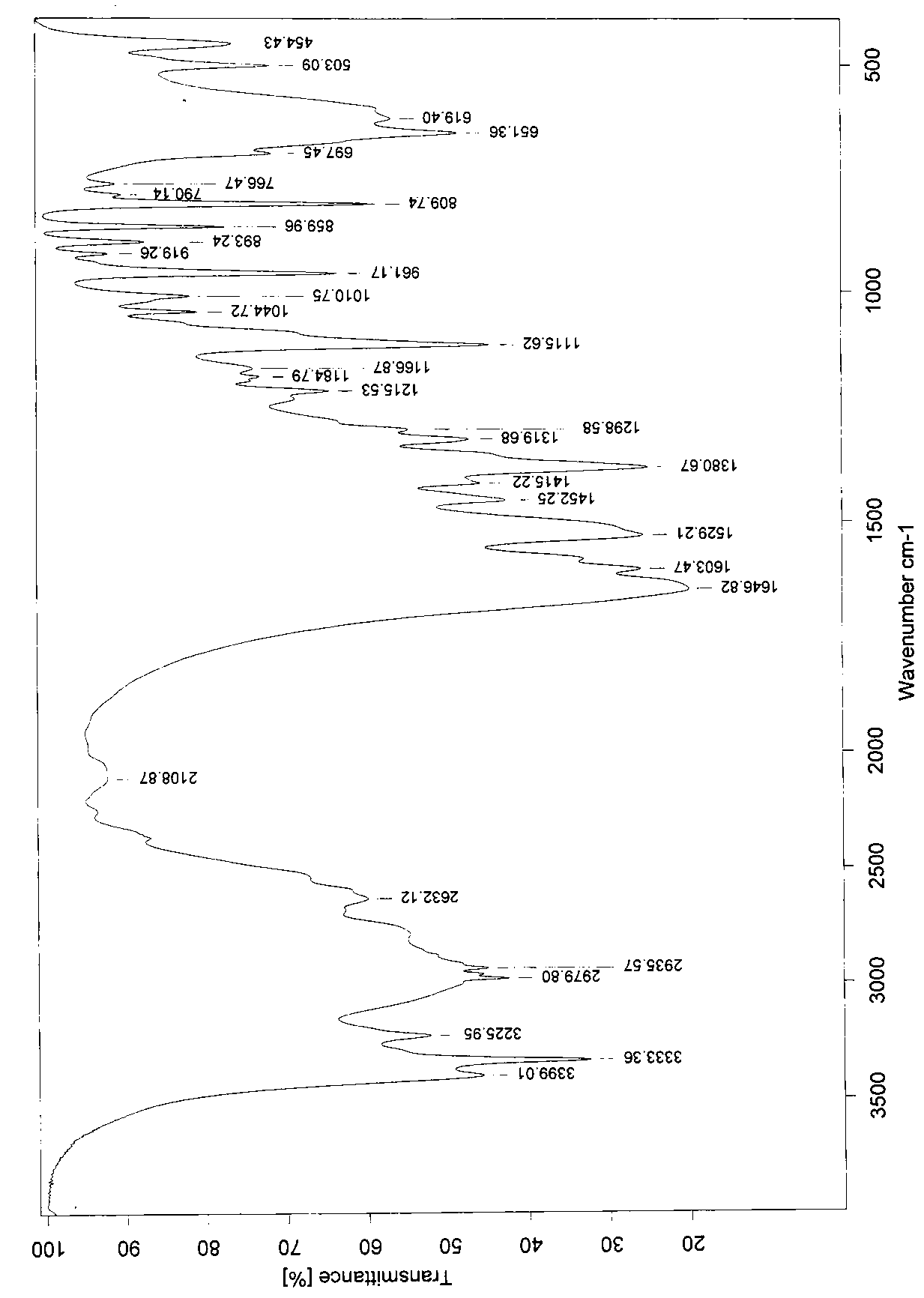
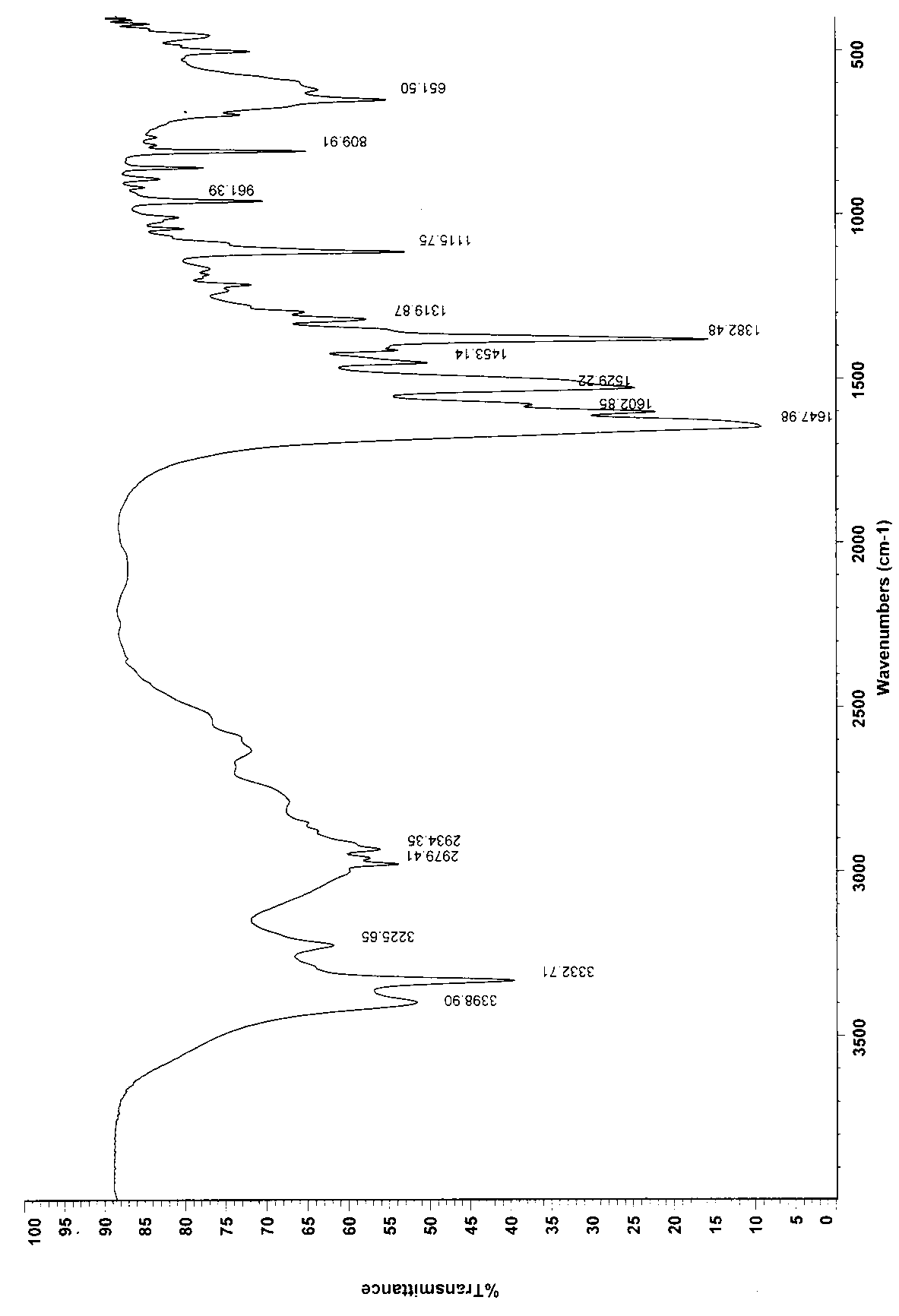
[0029] Weigh 400g of the crude product, add 1000ml of water to stir the solution to clarify, slowly add 4000ml of ethanol dropwise, stir at room temperature for 2h, filter, and dry the filter cake at 60°C to obtain 390g, yield 97.5%. Infrared identification spectrum see attached ...
Embodiment 3
[0031] Add 5000 mL of absolute ethanol into a 10L reaction kettle, stir, then add 694 g of N-phthaloyl-L-alanyl-L-glutamine, cool in an ice bath at 5°C~10°C, and pass in propylamine Gas to a pressure of 0.3~0.6MPa (molar ratio N-phthaloyl-L-alanyl-L-glutamine: propylamine: = 1:2), after the reaction at room temperature for 10h, 35 ℃ water bath to concentrate under reduced pressure Remove excess propylamine to PH=6~7, stand for crystallization for 10 h, filter, and dry the filter cake under normal pressure to obtain 420 g of white solid, yield 95%, HPLC: 98.9%.
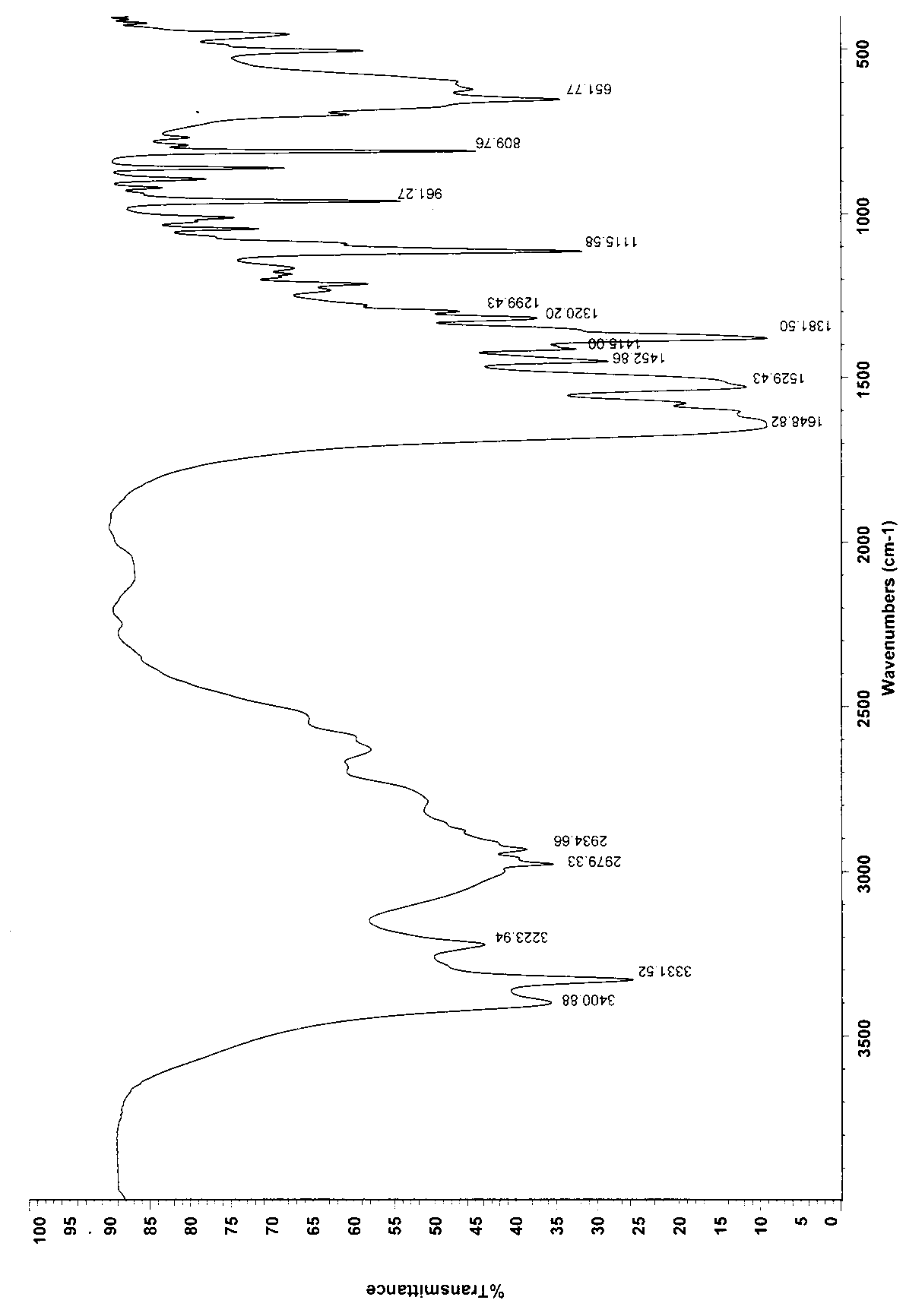
[0032] Weigh 400g of the crude product, add 1200ml of water to stir the solution to clarify, slowly add 3600ml of ethanol dropwise, stir at room temperature for 2h, filter, and dry the filter cake at 60°C to obtain 382g, yield 95.5%. Infrared identification spectrum see attached image 3 .
[0033] The L-alanyl-L-glutamine prepared in the embodiment of the present invention and the commercially available L-alanyl-L-g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com