Preparation method of chiral pantoprazole and sodium salt thereof
A technology of pantoprazole sodium and pantoprazole, which is applied in the field of preparation of chiral pantoprazole and its sodium salt, can solve the problem of insufficient optical purity and chemical purity of the product, complex post-treatment process, enantioselective The problem of low reactivity is solved, and the effects of simple post-treatment method, less solvent consumption and high enantioselectivity are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
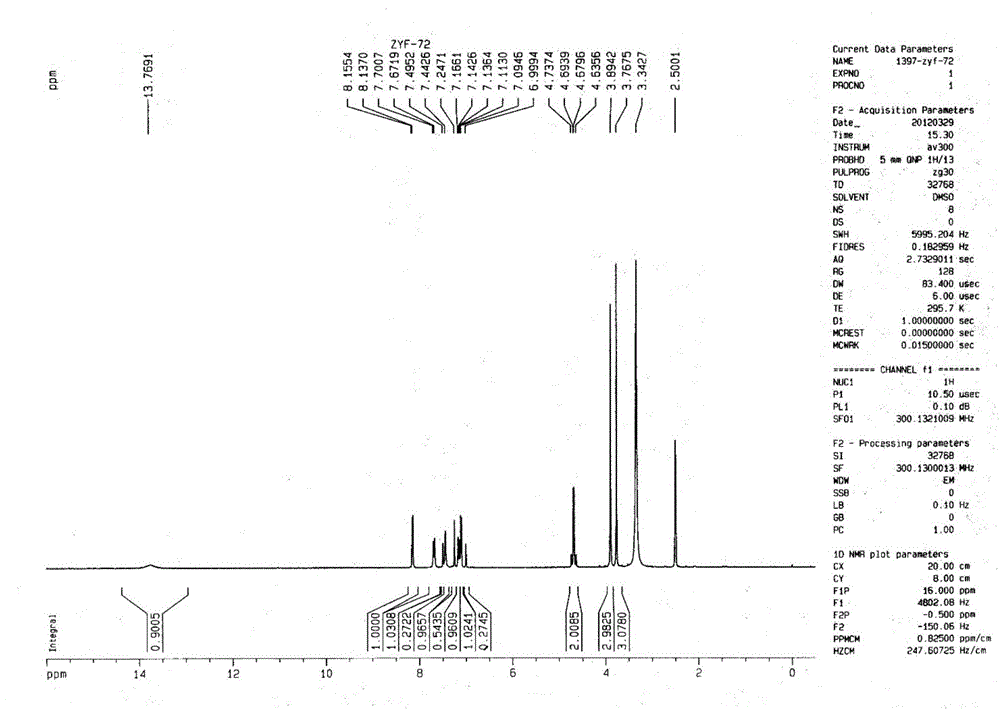
[0049] 3.674g 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)-methyl]-sulfanyl]-1H-benzimidazole (10mmol), 1.225g Mix N,N'-diethyl-(S,S)-tartrate diamide (6mmol) and 20mL toluene and heat up to 80°C (internal temperature); add 0.9mL tetraisopropoxytitanium (3mmol), keep at 80°C Stir for 1h; then add 54 μL of water (3mmol), and stir for 1h at 80°C; after cooling to 30°C, add 3.6mL of cumene hydroperoxide (20mmol), and react for 2h to terminate the reaction, and measure S-pantoprazole by HPLC The enantiomeric excess value is 91.87%, the conversion rate is 48.44%, and the content of peroxidation by-product sulfone is 0%.
Embodiment 2
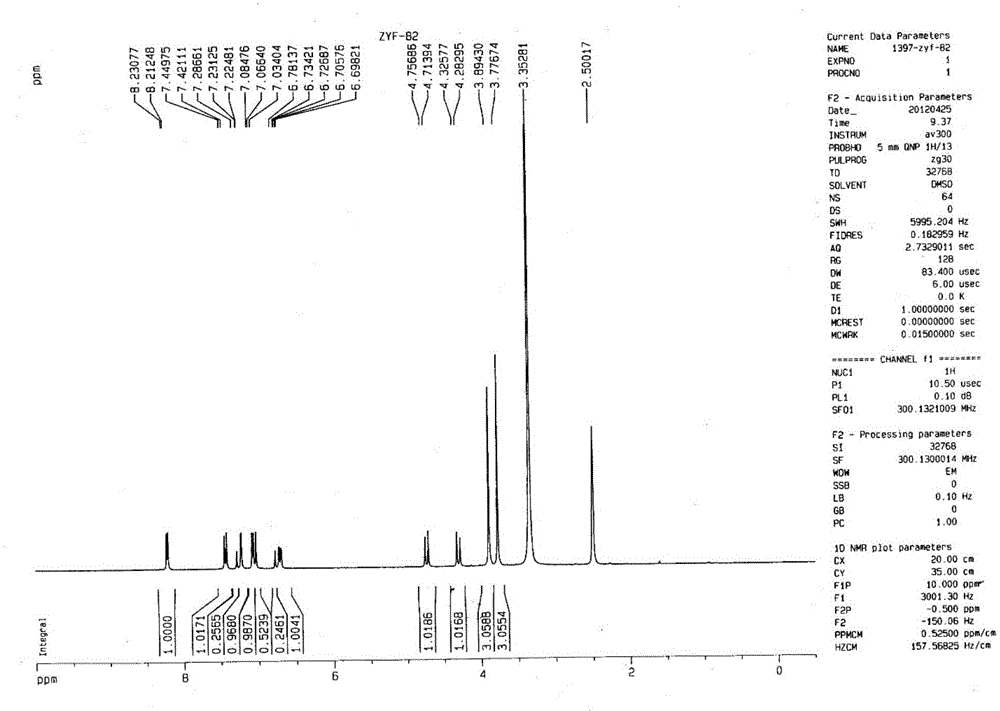
[0051] 3.674g 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)-methyl]-sulfanyl]-1H-benzimidazole (10mmol), 1.394g N,N'-di-n-propyl-(S,S)-tartaric acid diamide (6mmol) and 20mL toluene were mixed and heated to 80°C (internal temperature); 0.9mL tetraisopropoxytitanium (3mmol) was added, 80°C Keep stirring for 1 hour; then add 54 μL of water (3 mmol) and stir at 80°C for 1 hour; after cooling down to 30°C, add 3.6 mL of cumene hydroperoxide (20 mmol) and react for 2 hours to terminate the reaction. The enantiomeric excess of azole is 95.26%, the conversion rate is 36.65%, and the content of peroxidation by-product sulfone is 0.21%.
Embodiment 3
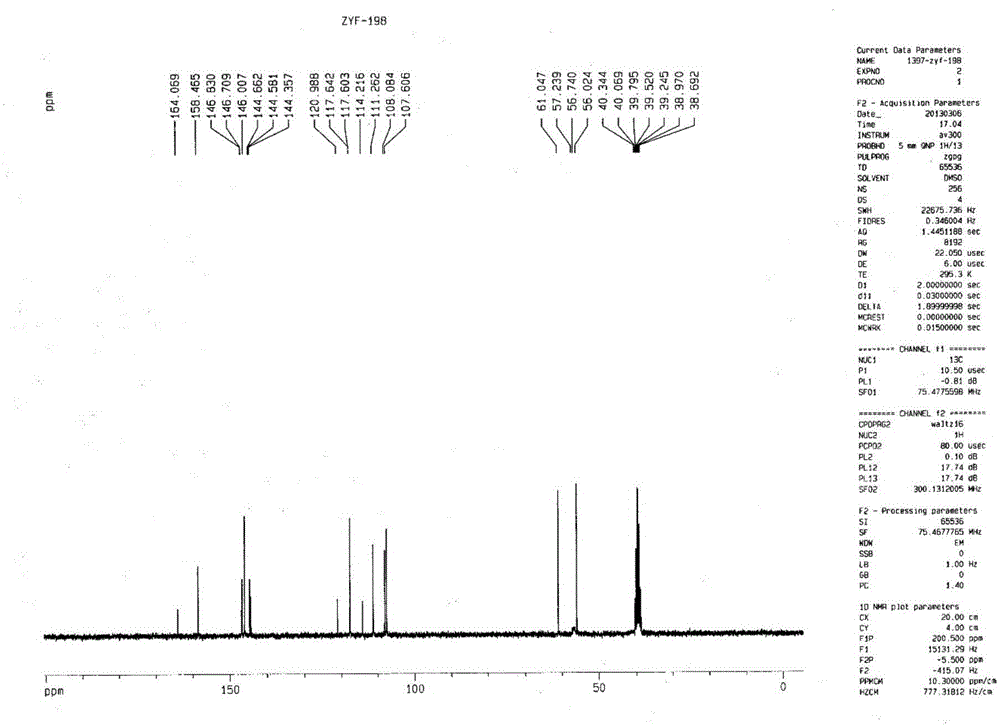
[0053] 3.674g 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)-methyl]-sulfanyl]-1H-benzimidazole (10mmol), 1.562g Mix N,N'-di-n-butyl-(S,S)-tartrate diamide (6mmol) and 20mL toluene and heat up to 80°C (internal temperature); add 0.9mL tetraisopropoxytitanium (3mmol), 80°C Keep stirring for 1 hour; then add 54 μL of water (3 mmol) and stir at 80°C for 1 hour; after cooling down to 30°C, add 3.6 mL of cumene hydroperoxide (20 mmol) and react for 2 hours to terminate the reaction. The enantiomeric excess of azole was 89.48%, the conversion rate was 28.12%, and the content of peroxidation by-product sulfone was 0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com