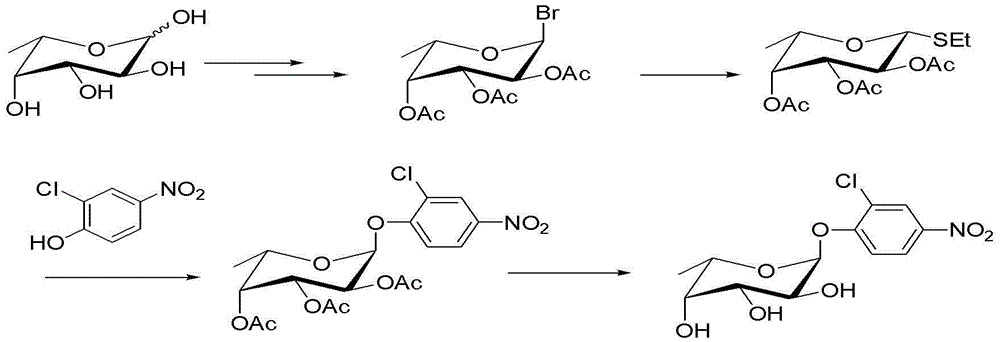
Method for preparing 2-chloro-4-nitrophenyl-alpha-L-fucoside
A technology of fucosides and nitrobenzene, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of low yield, unacceptable cost, and expensive starting materials. The effect of simple treatment and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Synthesis of 1-bromo-2,3,4-triacetyl-α-L-fucose:
[0023] Add 30ml of acetic anhydride to a 100ml three-necked bottle, turn on the electric stirrer, add 50mg of L-fucose to the three-necked bottle, if it is not fully dissolved, turn on the water bath and heat until the thermometer reads 40°C, slowly add 0.18g perchlorate with a 1ml syringe acid, the reaction liquid immediately becomes clear, and the reading on the thermometer rises. Remove the hot water bath immediately, keep the reading on the thermometer at about 40°C, and the upper and lower points do not exceed 2°C. Slowly add 7.5gL-fucose for about 40 minutes, and cool naturally to At room temperature, add 30ml of 30% hydrogen bromide acetic acid solution into the above-mentioned three-necked flask, continue to stir for 1.5 hours, TLC detects that the reaction is complete, pour it into 70ml of dichloromethane, wash twice with ice water (30ml×2), and then use Wash twice with 5% sodium bicarbonate (50ml×2)...
Embodiment 2
[0025] Example 2: Synthesis of 1-bromo-2,3,4-triacetyl-α-L-fucose:
[0026] Add 30ml of acetic acid to a 100ml three-necked bottle, turn on the electric stirrer, add 50mg of L-fucose to the three-necked bottle, turn on the water bath and heat until the thermometer reads 40°C, slowly add 0.5g of sulfuric acid with a 1ml syringe, and keep the thermometer reading At about 70°C, slowly add 7.5g of L-fucose for about 40 minutes, cool down to room temperature naturally, add 30ml of 30% hydrogen bromide acetic acid solution into the above-mentioned three-neck flask, continue to stir for 1.5 hours, TLC detects that the reaction is complete, pour into 70ml of dichloromethane, washed twice with ice water (30ml×2), then washed twice with 5% sodium bicarbonate (50ml×2), separated the organic phase, dried, and evaporated to dryness to obtain 16.53g of yellow syrup , recrystallized with ethanol to obtain 13.9 g of off-white solid, yield 85.5%.
[0027] LC-MS: [M] (positive ionization) = 35...
Embodiment 3
[0028] Example 3: Synthesis of 1-bromo-2,3,4-triacetyl-α-L-fucose:
[0029] Add 30ml of acetic anhydride to a 100ml three-necked bottle, turn on the electric stirrer, add 50mg of L-fucose to the three-necked bottle, turn on the water bath and heat until the thermometer reads 40°C, slowly add 2.5g of p-toluenesulfonic acid with a 1ml syringe, Remove the hot water bath, keep the thermometer reading at about 40°C, slowly add 7.5g of L-fucose for about 40 minutes, cool down to room temperature naturally, add 30ml of 30% hydrogen bromide propionic acid solution into the above three-necked bottle, continue Stir for 2.0 hours, TLC detects that the reaction is complete, cool to room temperature, pour into 70ml of dichloromethane, wash twice with ice water (30ml×2), then wash twice with 5% sodium bicarbonate (50ml×2), divide The organic phase was taken out, dried, and evaporated to dryness to obtain 14.86 g of yellow syrup, which was recrystallized with ethanol to obtain 12.6 g of off-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com