Pleuromutilin compound, preparation method of pleuromutilin compound, polymorphism and preparation method of polymorphism
A technology for pleuromutilin and compounds, applied in the field of drug synthesis, to achieve the effects of high reaction yield, simple synthesis process, and simple treatment method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
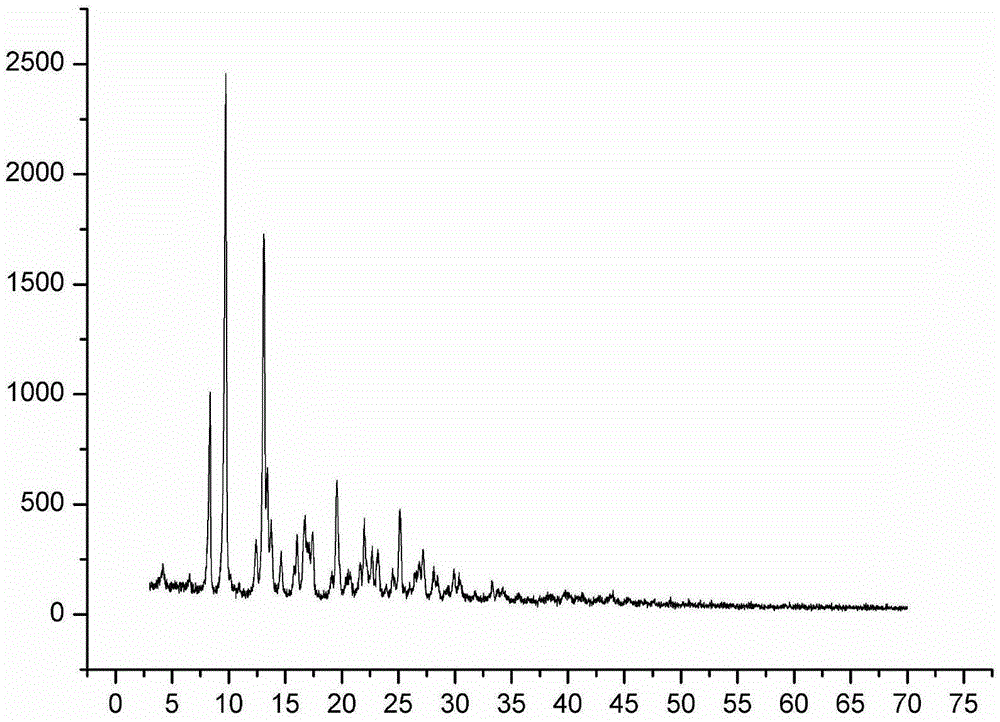
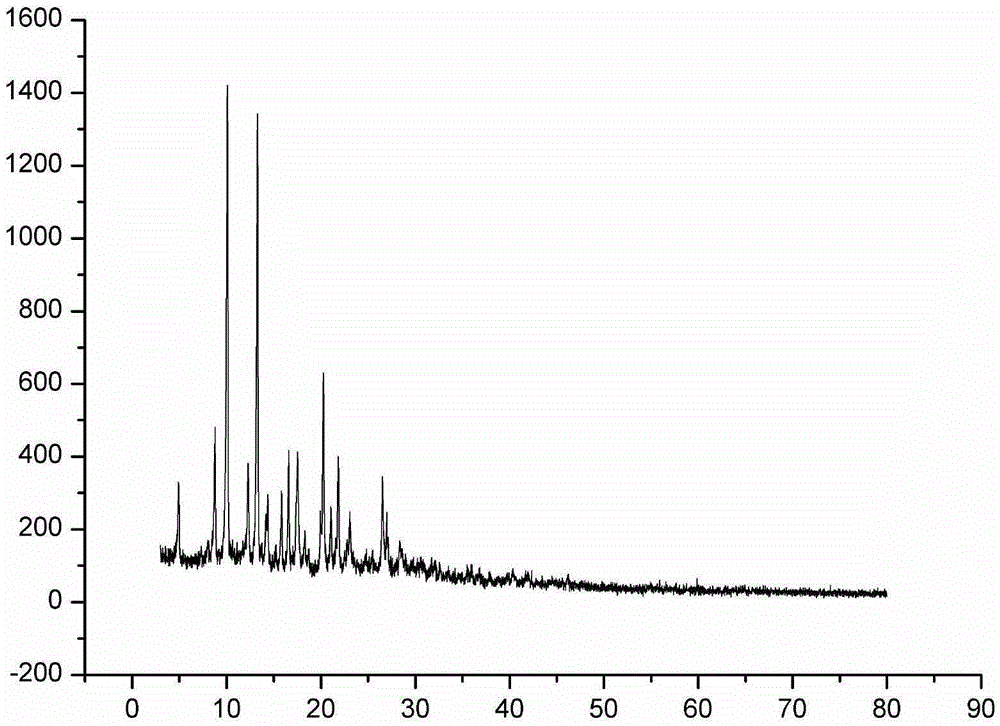
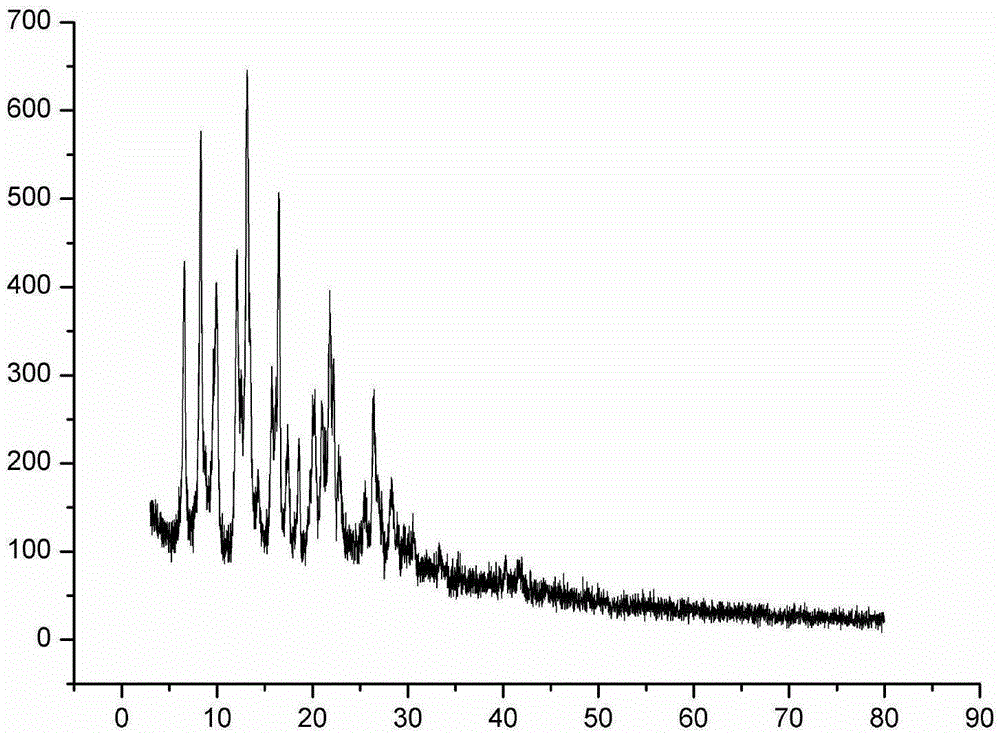
Image
Examples
Embodiment 1
[0045] Embodiment 1: A kind of pleuromutilin compound
[0046] A pleuromutilin compound, which is 14-O-[(4-amino-6-hydroxy-pyrimidin-2-yl) mercaptoacetyl] Mutilin formula (i), and 14-O-[(4 -Amino-6-keto-pyrimidin-2-yl)thioacetyl]Multiline (formula ii), wherein 14-O-[(4-amino-6-keto-pyrimidin-2-yl)thioacetyl] Mutilin (formula ii) is another conformation of 14-O-[(4-amino-6-hydroxy-pyrimidin-2-yl)thioacetyl] Mutilin formula (i).
Embodiment 2
[0048] The preparation method of pleuromutilin compounds described in Example 1: 4.45 g of pleuromutilin (0.01 mol) with a purity of 85%, 2.10 g of p-toluenesulfonyl chloride (0.01 mol) and 40 mL of methyl iso Butanone (methylisobutylketone, MIBK) was added into a 100mL three-necked flask, heated and stirred to dissolve pleuromutilin and p-toluenesulfonyl chloride. When the temperature rose to 60°C, 2.2mL of 10N NaOH aqueous solution was added dropwise, and the reaction 30min; then the pH value of the reaction solution was adjusted to 8-9 with 5N hydrochloric acid, and 1.65g of 4-amino-6-hydroxy-2-mercaptopyrimidine sodium salt (0.01mol) (4-amino-6-hydroxy-2-mercapto The preparation method of the sodium salt of pyrimidine is to dissolve NaOH in methanol, add dropwise 4-amino-6-hydroxy-2-mercaptopyrimidine, and stir for 30 minutes to obtain), continue the reaction at 60°C for 5 hours, and evaporate to dryness under reduced pressure Add 20mL of water and 20mL of tert-butyl methy...
Embodiment 3
[0051] The preparation method of pleuromutilin compounds described in Implementation 1: 4.45 g of pleuromutilin (0.01 mol) with a purity of 85%, 2.10 g of p-toluenesulfonyl chloride (0.01 mol) and 40 mL of methyl isobutyl Add ketone (methylisobutylketone, MIBK) into a 100mL three-necked flask, heat up, and stir to dissolve pleuromutilin and p-toluenesulfonyl chloride. When the temperature rises to 50°C, add 2.2mL of 10N NaOH aqueous solution dropwise, and react for 60min ; Then the reaction solution was adjusted to pH 8-9 with 5N hydrochloric acid, and 1.65 g of 4-amino-6-hydroxyl-2-mercaptopyrimidine sodium salt (0.01mol) was added (4-amino-6-hydroxyl-2-mercaptopyrimidine The preparation method of the sodium salt is to dissolve NaOH in methanol, dropwise add 4-amino-6-hydroxy-2-mercaptopyrimidine, and stir for 30 minutes to obtain), continue to react at 50°C for 6 hours, and evaporate MIBK to dryness under reduced pressure , add 20mL of water and 20mL of tert-butyl methyl eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com