A kind of preparation method of prothioconazole intermediate
A technology for prothioconazole and intermediates is applied in the field of preparation of prothioconazole intermediates, and can solve the problems of decreased product yield and purity, complicated post-processing, large waste liquid and solid waste, and the like, and achieves shortened reaction time, The post-processing method is simple and the three wastes are less.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
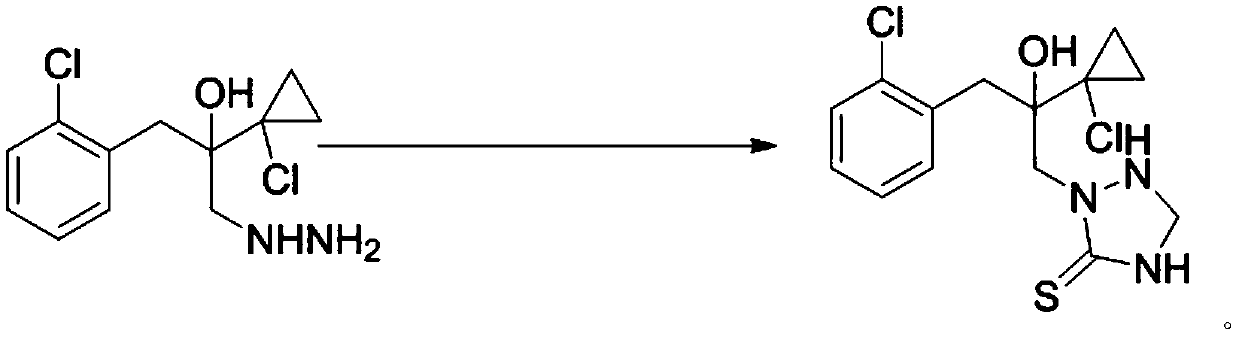
[0033] Dissolve 44 grams of [1-(2-chlorophenyl)-2-(1-chlorocyclopropyl)-2-hydroxyl]-propylhydrazine in 80mL of toluene, keep it warm at 30°C, and slowly add it dropwise with a mass concentration of 37 % formaldehyde aqueous solution 15 grams, drop completely in about 30 minutes, then add 12.2 grams of ammonium thiocyanate, slowly drip the hydrochloric acid (35 grams) that mass concentration is 30%, add dropwise completely in about 30 minutes, keep warm for one hour, GC It was detected that the reaction was complete, the system was lowered to 20°C for recrystallization, and the product 2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1, About 50 g of 2,4-triazolidine-5-thione-1-yl)-propane (purity>95%), yield 90%.
[0034] Product m.p:152-154℃. 1 H NMR (400MHz, CDCl 3)δ7.65-7.47(m,1H),7.43-7.31(m,1H),7.26-7.09(m,2H),6.11(s,1H),5.11(t,J=11.2Hz,1H),4.58 (d, J=12.3Hz, 2H), 4.48(dd, J=12.8, 6.7Hz, 1H), 4.17(s, 2H), 3.63(d, J=14.0Hz, 1H), 3.08(d, J= 14.0Hz,1H),1.32...
Embodiment 2
[0036] Dissolve 44 grams of [1-(2-chlorophenyl)-2-(1-chlorocyclopropyl)-2-hydroxyl]-propylhydrazine in 80mL of toluene, keep it warm at 33°C, and slowly add it dropwise with a mass concentration of 37 % formaldehyde aqueous solution 15 grams, drop completely in about 30 minutes, then add 13 grams of sodium thiocyanate, slowly add hydrochloric acid (35 grams) with a mass concentration of 30%, drop completely in about 30 minutes, keep warm for one hour, GC It was detected that the reaction was complete, the system was lowered to 20°C for recrystallization, and the product 2-(1-chloro-cyclopropan-1-yl)-1-(2-chlorophenyl)-2-hydroxyl-3-(1, About 51 g of 2,4-triazolidine-5-thione-1-yl)-propane (purity>95%), yield 92%.
Embodiment 3
[0038] Dissolve 44 grams of [1-(2-chlorophenyl)-2-(1-chlorocyclopropyl)-2-hydroxyl]-propylhydrazine in 80mL of toluene, keep it warm at 35°C, and slowly add it dropwise with a mass concentration of 37 % paraformaldehyde aqueous solution 13 grams (4.81 grams of paraformaldehyde solids are dissolved in 8.19 grams of water), drop completely in about 30 minutes, then add potassium thiocyanate 15.6 grams, slowly add mass concentration and be 30% hydrochloric acid (35 gram), about 30 minutes to drop completely, keep warm for one hour, GC detects that the reaction is complete, the system is reduced to 20 ° C for recrystallization, and the product 2-(1-chloro-cyclopropan-1-yl)-1-(2 About 51 g of -chlorophenyl)-2-hydroxy-3-(1,2,4-triazolidine-5-thiol-1-yl)-propane (purity>95%), yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com