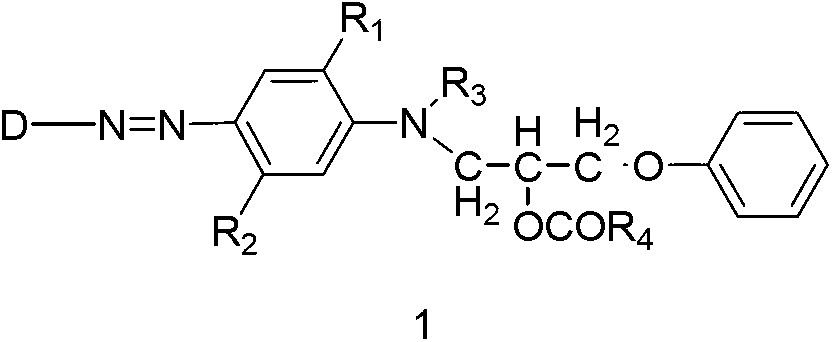
Easter compounds and preparation method thereof
A technology of ester compounds and compounds, applied in the field of ester compounds and their preparation, can solve the problems of high toxicity of raw materials, complicated preparation process, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] The preparation of embodiment 1 compound 6
[0148]
[0149] At room temperature, add 67 parts by weight of 30% sodium hydroxide aqueous solution into a three-necked flask, then add 47 parts by weight of phenol and 5 parts by weight of PEG-400 under stirring, stir evenly, and then use dropwise Slowly add 66 parts of heavy epichlorohydrin into the funnel dropwise for about 1-2 hours. After the dropwise addition, slowly raise the temperature to 50°C-55°C for reaction. After 3h-5h, the raw materials react completely, add 50mL of water to fully dissolve the solid, and then Stand for stratification, remove the water phase, add 100mL of water for washing, and adjust the pH to neutral with a small amount of dilute hydrochloric acid, stir well and then stand for stratification, collect the oil phase, and distill under reduced pressure to remove excess epichlorohydrin and water. 52 g of the compound of the following formula was obtained, the yield was 70.5%, and the HPLC pur...
Embodiment 2
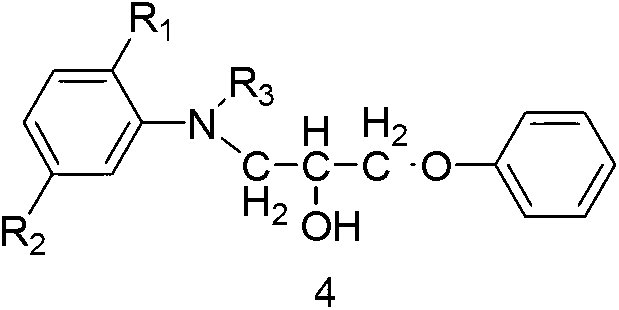
[0150] Example 2 Compound 4 (R 1 for H, R 2 for H, R 3 for the preparation of ethyl)
[0151] Add 18 parts by weight of N-ethylaniline and 30 parts by weight of acetic acid into a three-necked flask, stir evenly and then slowly raise the temperature to 50°C, then slowly add 24 parts by weight of compound 6 dropwise from a constant pressure dropping funnel for about 3 hours ~ 4h, after the addition, keep it warm at 50°C for 3h ~ 4h to complete the reaction, and compound 4 (R 1 for H, R 2 for H, R 3 Ethyl) in acetic acid solution was directly used in the next reaction. Yield 97.3%, HPLC purity 92.4%, LC-MS (ESI): [M+H] + 272.2, [M+Na] + 294.2.
[0152]
Embodiment 3
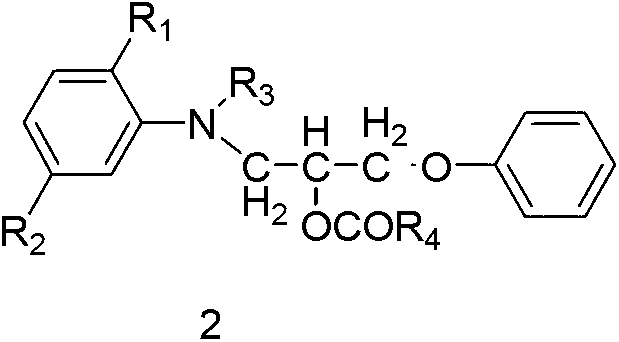
[0153] Embodiment 3 ester compound 2-1 (R 1 for H, R 2 for H, R 3 is ethyl, R 4 for the preparation of methyl)
[0154] 29 parts of heavy compound 4 (R 1 for H, R 2 for H, R 3 Ethyl) into a three-necked flask, slowly warm up to 50°C, at this temperature, slowly add 13 parts of heavy acetic anhydride dropwise from a constant pressure dropping funnel, dropwise for about 2 hours, and keep warm at 50°C until the reaction of the raw materials is complete , to obtain ester compound 2-1 (R 1 for H, R 2 for H, R 3 is ethyl, R 4 Methyl) in acetic acid solution, yield 96.1%, HPLC purity 94.3%, LC-MS (ESI): [M+H] + 314.4, [M+Na] + 336.4.
[0155]
[0156] The ester compounds 2-1 to 2-12 were prepared according to the methods of Examples 1 to 3, and their relevant experimental data and structure identification data are shown in Table 1.
[0157] Table 1 Experimental data and structural identification data of compounds 2-1~2-12
[0158]
[0159]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com