Nano spice precursor based on 2-carbonyl acetate and application thereof
A carbonyl acetate and perfume technology, applied in the field of nano-perfume precursors based on 2-carbonyl acetate, can solve the problems of difficult removal and long reaction time, and achieve short reaction time, few synthesis steps and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
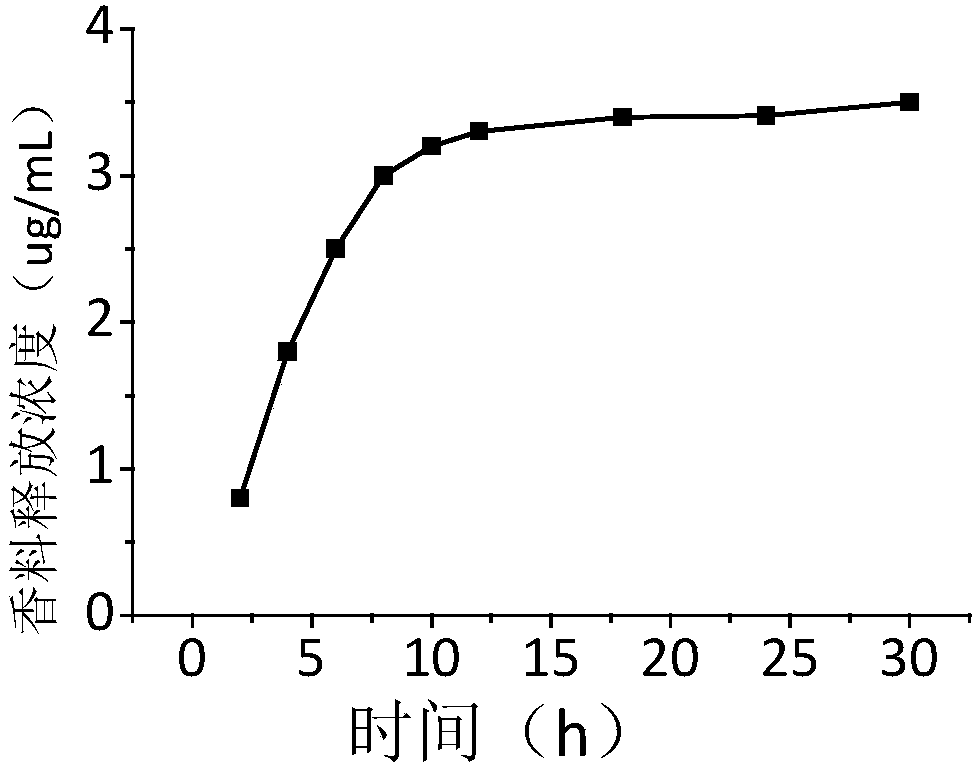
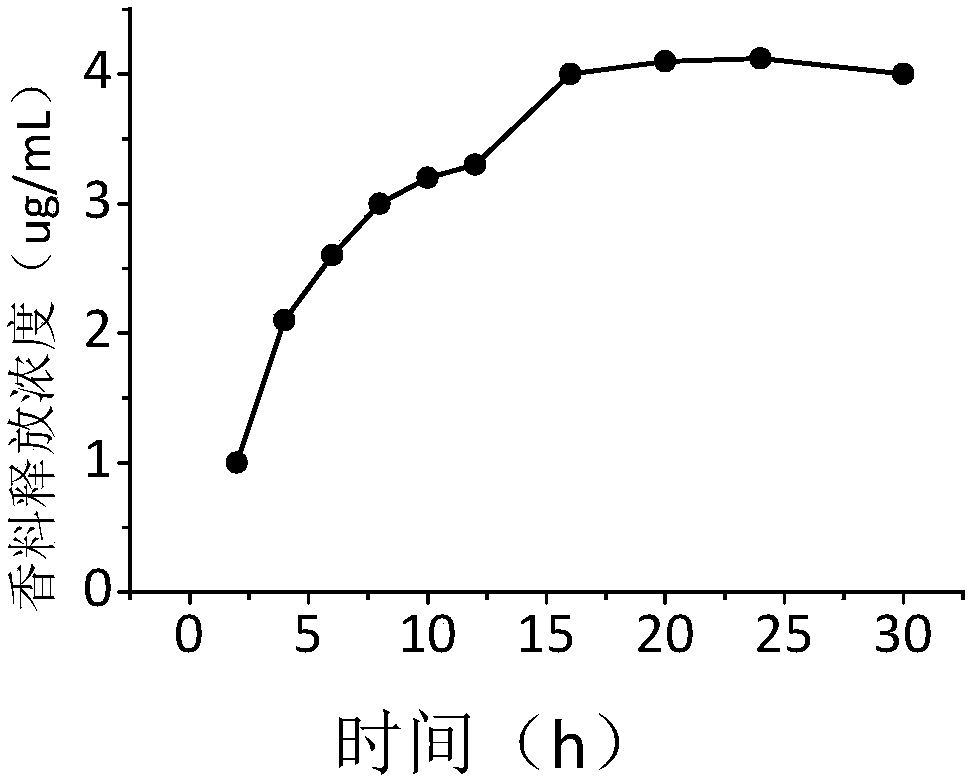
Image
Examples
Embodiment 1
[0070] R = isobutyl
[0071] (1) Synthesis of compound shown in formula II-1
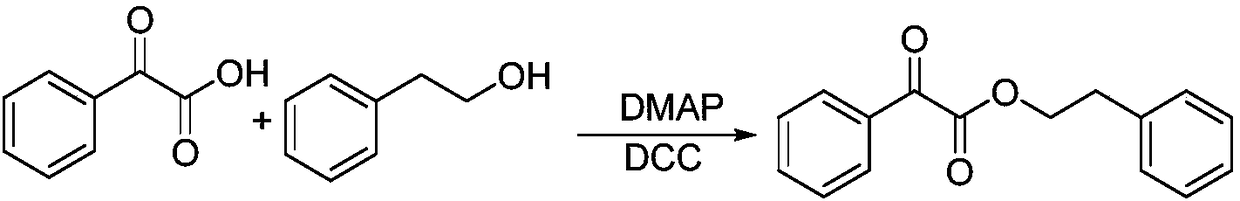
[0072] At room temperature, add oxalyl chloride (12.69g, 8.5mL, 100mmol) into the three-necked flask, add phenylethanol (1.22g, 10mmol) with a syringe, continue the reaction for 1h after the addition, stop stirring, and immediately remove the excess oxalyl chloride by rotary evaporation , to obtain compound II-1. The structure is as follows:
[0073]
[0074] (2) Synthesis of compound I-1
[0075] At room temperature, 10 mL of dichloromethane, potassium carbonate (35 mg, 0.25 mmol), 1-aminopropyl isobutyl cage polysilsesquioxane (Aminopropyl lsobutyl POSS) (0.44 g, 0.5 mmol) were successively added into a three-necked flask. ), stirred evenly, slowly added the compound II-1 (160mg, 0.75mmol, 1.5equiv) obtained in step (1) into the flask dropwise, after the dropwise addition was completed, reacted at room temperature for 1h, and after the reaction was completed, the solvent was removed by rot...
Embodiment 2
[0087] R = isobutyl
[0088] (1) Synthesis of compound shown in formula II-2
[0089] At room temperature, add oxalyl chloride (12.69g, 8.5mL, 100mmol) into a three-neck flask, add tetrahydrogeraniol (1.58g, 10mmol) with a syringe, continue the reaction for 1h after the addition, stop stirring, and immediately remove the excess The oxalyl chloride obtained compound II-2, the structure is as follows:
[0090]
[0091] (2) Synthesis of Compound I-2
[0092] At room temperature, 10 mL of dichloromethane, potassium carbonate (35 mg, 0.25 mmol), 1-aminopropyl isobutyl cage polysilsesquioxane (Aminopropyl lsobutyl POSS) (0.44 g, 0.5 mmol) were successively added into a three-necked flask. ), stirred evenly, and slowly added compound II-2 (186mg, 0.75mmol, 1.5equiv) obtained in step (1) into the flask dropwise. After the dropwise addition, reacted at room temperature for 1h. . Then take 5mL of methanol with a measuring cylinder, wash the white solid in the bottle, and filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com