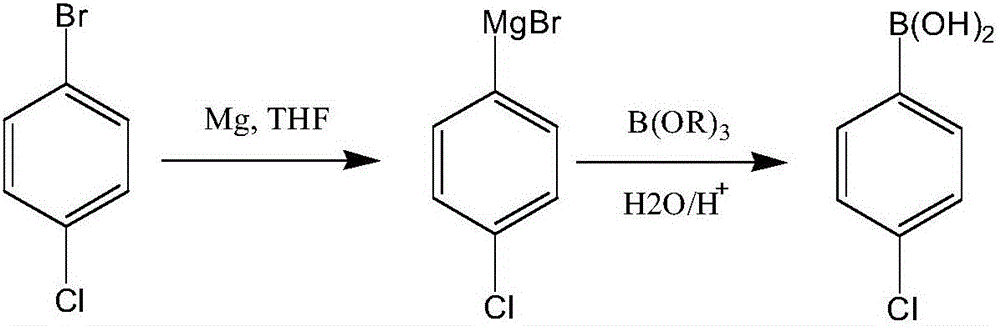
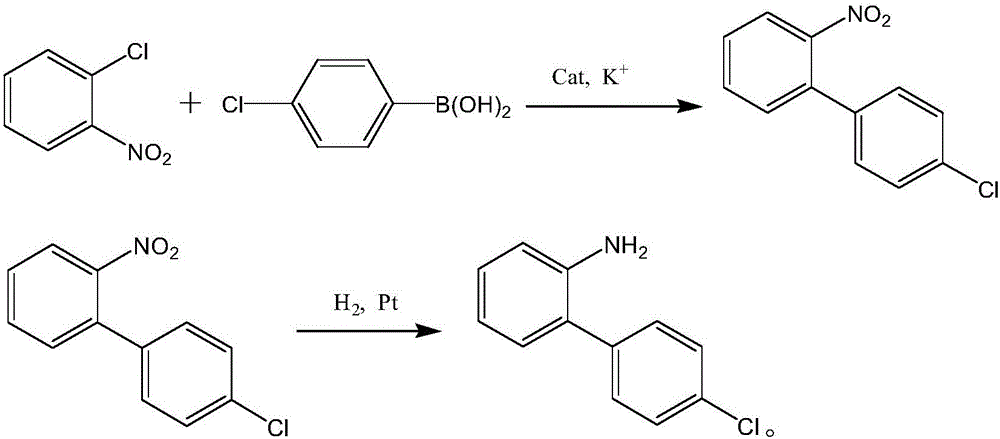
Synthesis process of boscalid intermediate 2-(4-chlorphenyl)phenylamine
A technology for the synthesis of boscalid and boscalid, which is applied in the field of synthesis of boscalid intermediate 2-aniline, which can solve the problems of insufficient synthesis, low work efficiency, and low product yield, and achieve excellent workmanship and high work efficiency. High, guaranteed high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under the protection of stirring and inert gas, put tert-butanol, water and potassium tert-butoxide into a stirred reaction vessel and stir fully to form a mixed solvent to be reacted, take out 100ml mixed solvent and add 30g o-chloronitrobenzene and 33g p-chloro Phenylboronic acid, stir well until the particles are all dissolved, continue to add a total of 1.5mg of Pd(OAc) to the mixed solvent 2 , 0.3mg of KI-based catalyst, fully stirred the temperature to 60°C and reacted for 4h, then cooled to 35°C, filtered to obtain the filtrate, and added 0.75mg of Pt-based catalyst to the filtrate, adjusted the temperature to 60°C and continued Hydrogen gas was introduced, and the finally obtained reaction solution was concentrated to 80° C. and cooled to obtain 35 g of finished product 2-(4-chlorophenyl)aniline, the content of which was 93.4% after detection.
Embodiment 2
[0031] Under the protection of stirring and inert gas, put tert-butanol, water and potassium tert-butoxide into a stirred reaction vessel and stir fully to form a mixed solvent to be reacted, take out 120ml mixed solvent and add 30g o-chloronitrobenzene and 35g p-chloro Phenylboronic acid, stir well until the particles are all dissolved, and continue to add a total of 3.5 mg of Pd(OAc) to the mixed solvent 2 , 0.6mg of KI-based catalyst, fully stirred the temperature to 80°C and reacted for 6h, then cooled to 40°C, filtered to obtain the filtrate, and added 1.75mg of Pt-based catalyst to the filtrate, adjusted the temperature to 80°C and continued Hydrogen gas was introduced, and the finally obtained reaction solution was concentrated to 70° C. and lowered in temperature to obtain 36 g of finished 2-(4-chlorophenyl)aniline, the content of which was 93.9% after detection.
Embodiment 3
[0033] Under the protection of stirring and inert gas, put tert-butanol, water and potassium tert-butoxide into a stirring reaction vessel and stir fully to form a mixed solvent to be reacted, take out 150ml mixed solvent and add 30g o-chloronitrobenzene and 40g p-chloro Phenylboronic acid, stir well until the particles are all dissolved, and continue to add a total of 3.5 mg of Pd(OAc) to the mixed solvent 2 , 0.3mg of KI-based catalyst, fully stirred the temperature to 90°C and reacted for 8h, then cooled to 45°C, filtered to obtain the filtrate, and added 1.75mg of Pt-based catalyst to the filtrate, adjusted the temperature to 90°C and continued Hydrogen gas was introduced, and the finally obtained reaction solution was concentrated to 90° C. and cooled to obtain 40 g of finished product 2-(4-chlorophenyl)aniline, the content of which was 94.5% after detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com