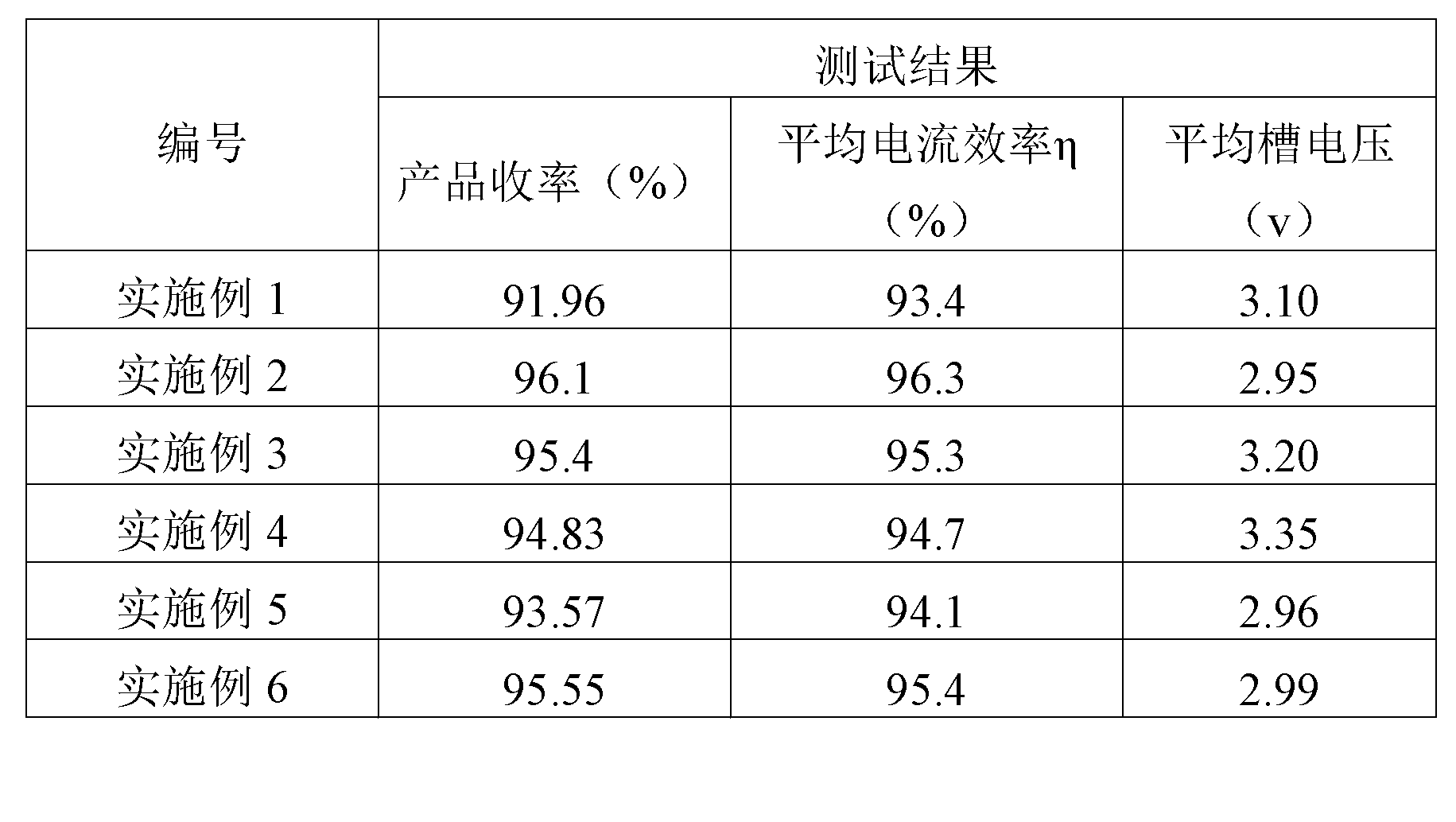
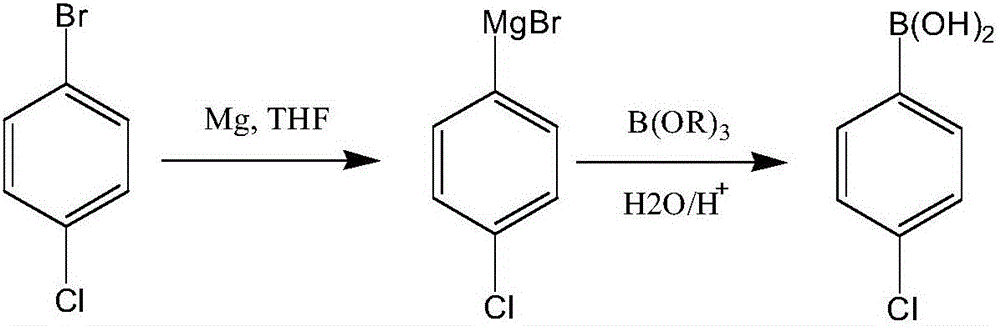
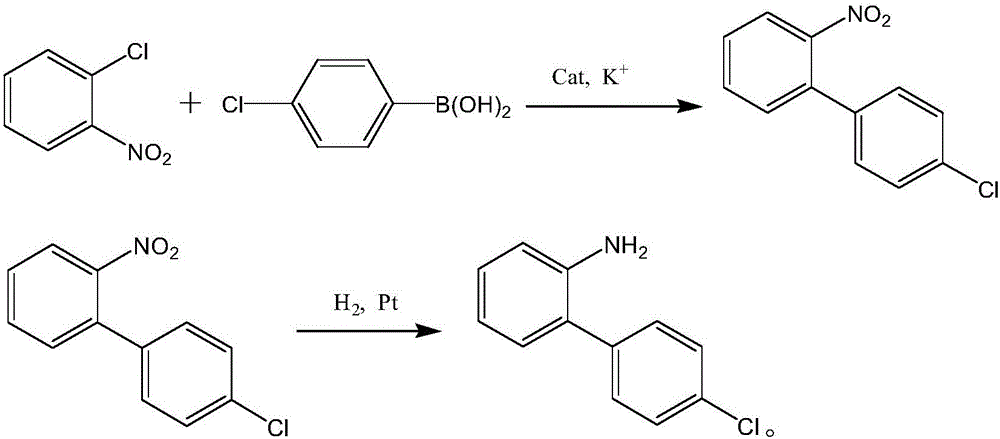
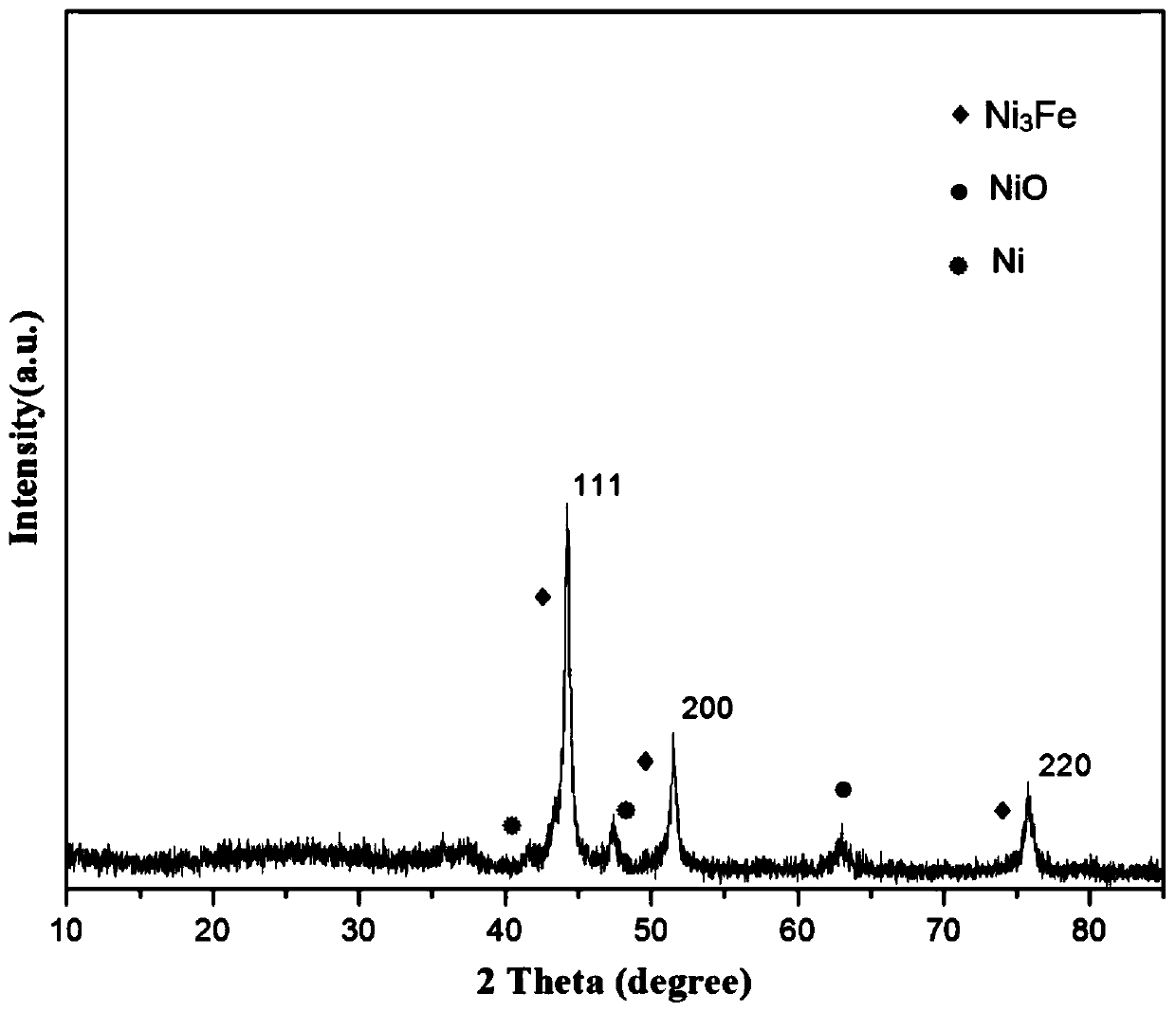
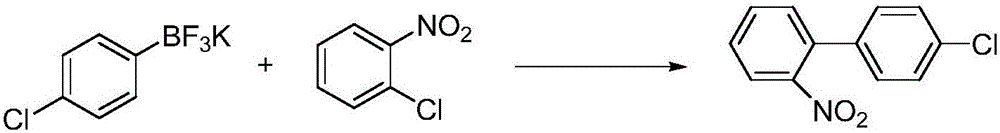Patents
Literature
58 results about "O-chloronitrobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
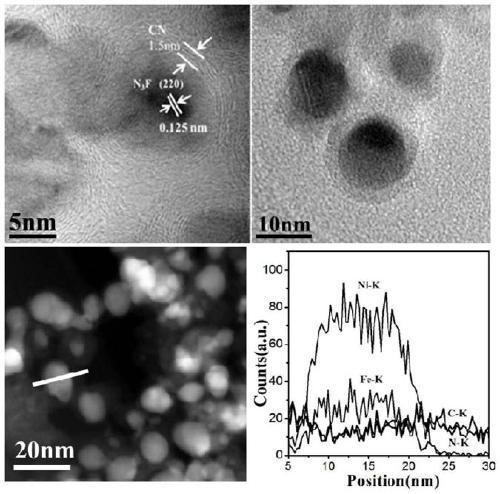
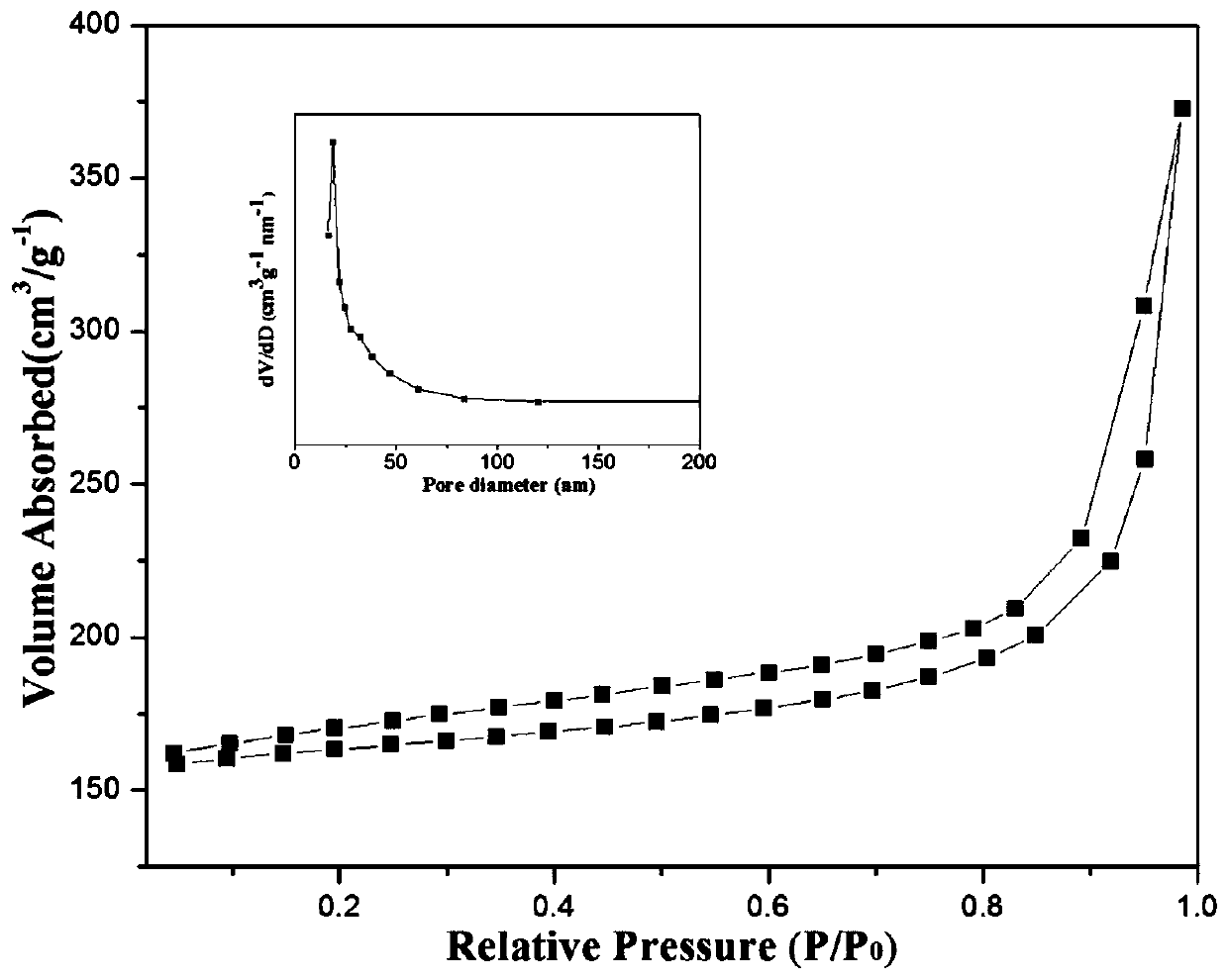
Preparation of nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst and application thereof in catalyzing o-chloronitrobenzene hydrogenation reaction
ActiveCN106732733AMaterial nanotechnologyPhysical/chemical process catalystsNano catalystNitro compound
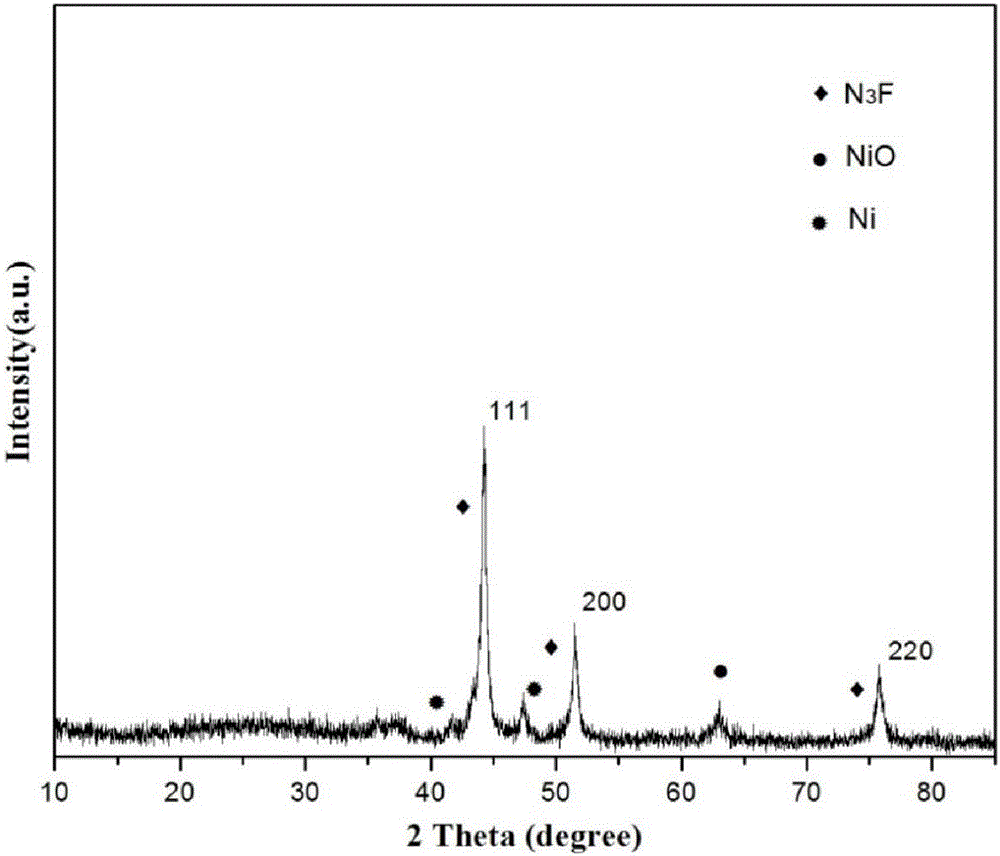
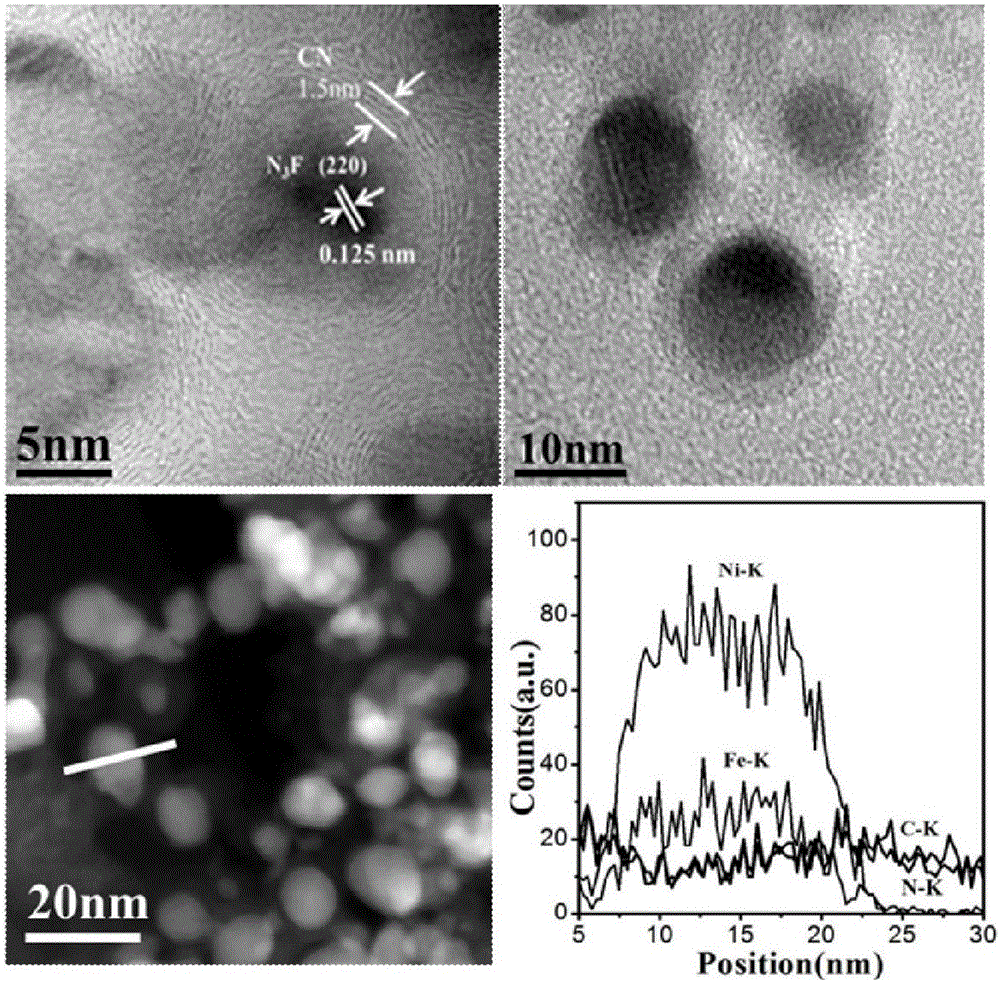
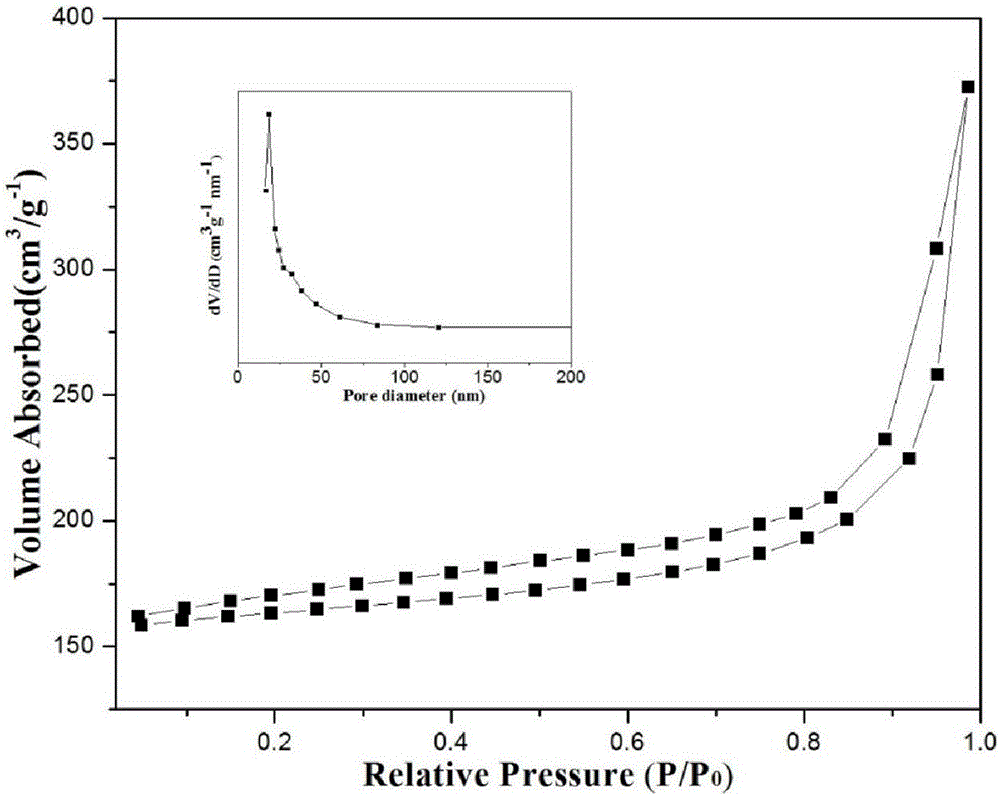
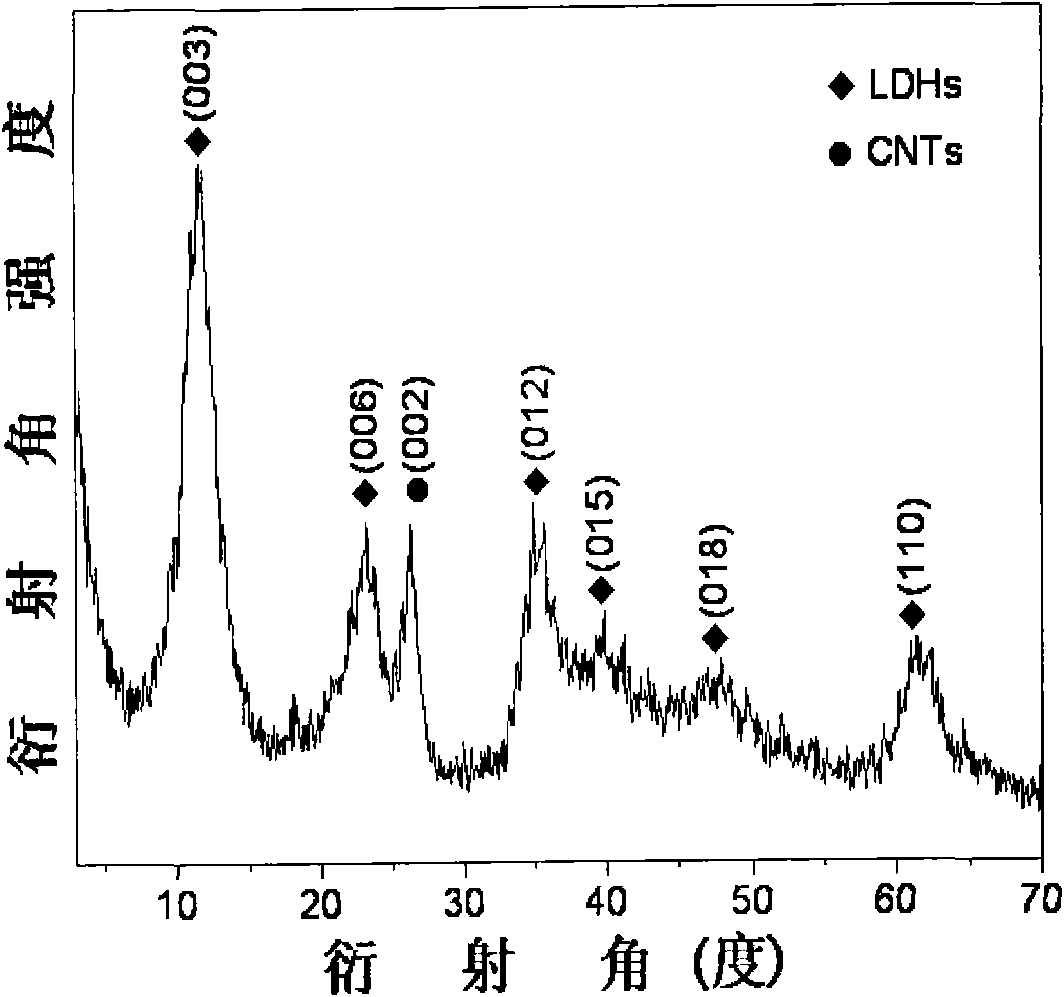
The invention provides a preparation method of a nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst and the application of the nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst in catalyzing an o-chloronitrobenzene hydrogenation reaction. According to the method, the novel nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst is prepared by synthesizing a ferronickel layered doubled hydroxide precursor with small grain size and high surface energy through a nucleation crystallization isolation method, evenly mixing the ferronickel layered doubled hydroxide precursor with a melamine and dicyandiamide mixed carbon material precursor, and finally self-reducing at high temperature. The nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst is efficiently applied to the reaction where halogenated aniline is generated through catalytic hydrogenation of a nitro-halogen compound, and the conversion rate of o-chloronitrobenzene and the selectivity of o-chloroaniline are respectively up to 95-100% and 98-100%. The structure of the novel nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst is unique and novel, the process is green and energy-saving, the structure of the catalyst is stable, and the catalyst has a broad application prospect.
Owner:BEIJING UNIV OF CHEM TECH
Production process of catalytically hydrogenating nitrobenzene halide to synthesize haloarylamine
InactiveCN1506348AInhibition of hydrodehalogenationHigh catalytic activityOrganic compound preparationAmino compound preparationHydrogenation reactionNitrobenzene
The production process is liquid phase catalytic hydrogenation reaction of nitrobenzene halide, such as o-chloronitrobenzene, p-chloronitrobenzene, 3, 4-dichloronitrobenzene and 3-chloro-4-fluoronitrobenzene, etc. with carbon nanotube carried Pd and Pt as hydrogenation catalyst to produce corresponding haloarylamine. The said production process can inhibit hydrodehalogenation, and has the advantages of high selectivity, high yield and high catalyst stability, etc. and thus high practical value.
Owner:ZHEJIANG UNIV OF TECH
High dispersed loaded nano-metal Ni catalyst and preparation method thereof
InactiveCN102068991AFirmly assembledGuaranteed smooth assemblyMaterial nanotechnologyOrganic compound preparationCarbon nanotubeParticle-size distribution
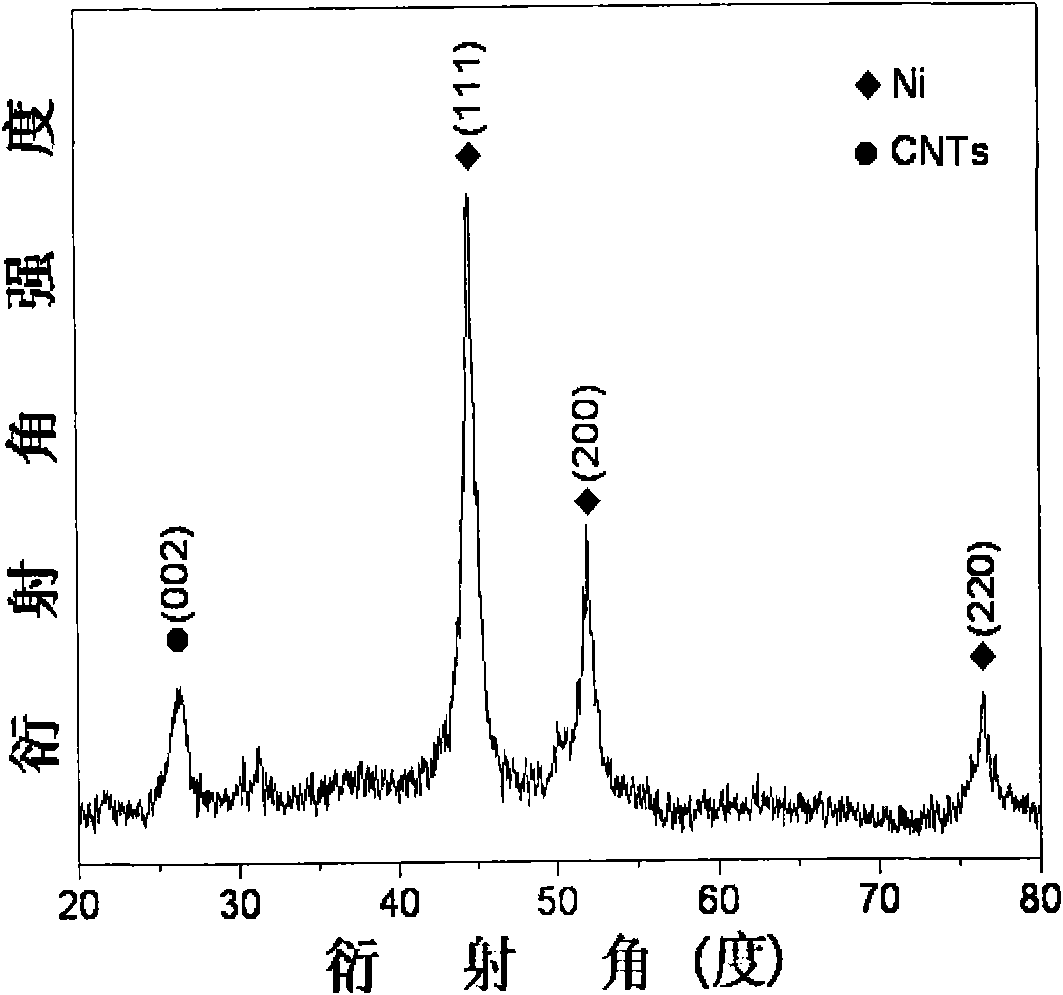
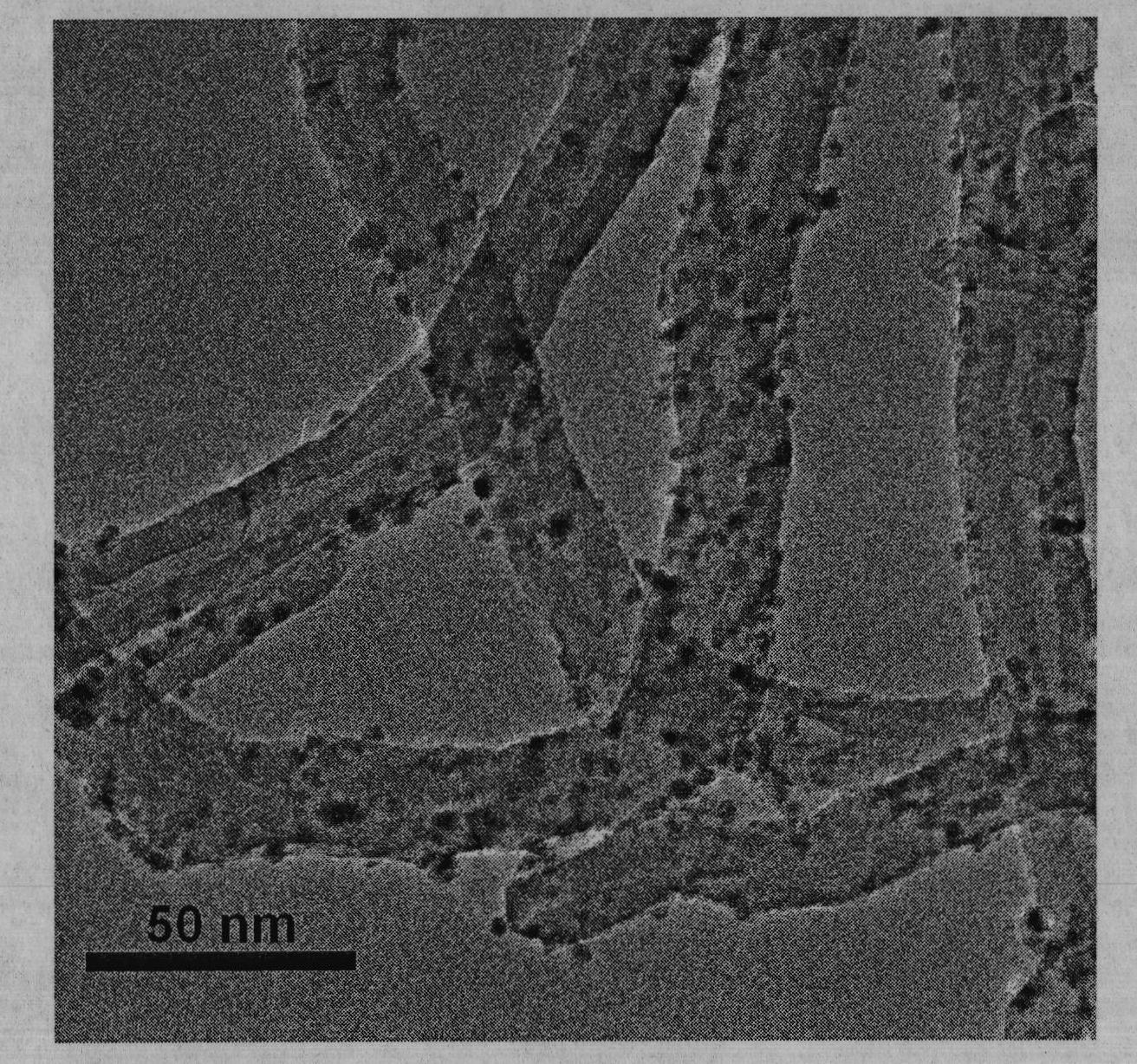
The invention provides a high dispersed loaded nano-metal Ni catalyst and a preparation method thereof, and belongs to the technical field of preparation of metal nano-particles. The bridging role of L-cysteine is utilized, the co-precipitation method is adopted for firstly preparing a NiAl-layered double-metal hydroxide / polyacrylic acid surface functional carbon nano-tube complex precursor, and then the high dispersed Ni metal catalyst loaded by carbon nano-tubes is further obtained by reducing the precursor through hydrogen. The loaded nano-metal Ni catalyst is formed by uniformly loading the mixture of Ni nano-particles and amorphous Al2O3 on the surface of the carbon nano-tubes, wherein the weight percentage content of the Ni is 3-30%, the weight percentage content of the amorphous Al2O3 is 1-10%, and the weight percentage content of the carbon nano-tubes is 60-95%; and the particle size distribution of the Ni nano-particles is 6-12nm. The catalyst is applied in hydrogenation reaction of o-chloronitrobenzene and can enable the o-chloronitrobenzene to perform selective hydrogenation for further generating o-chloroaniline, thereby showing good catalytic hydrogenation performance.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing o-chloroaniline
InactiveCN1660774AHigh yieldLow costOrganic compound preparationAmino compound preparationAlcoholSolvent
Owner:ZHEJIANG UNIV OF TECH
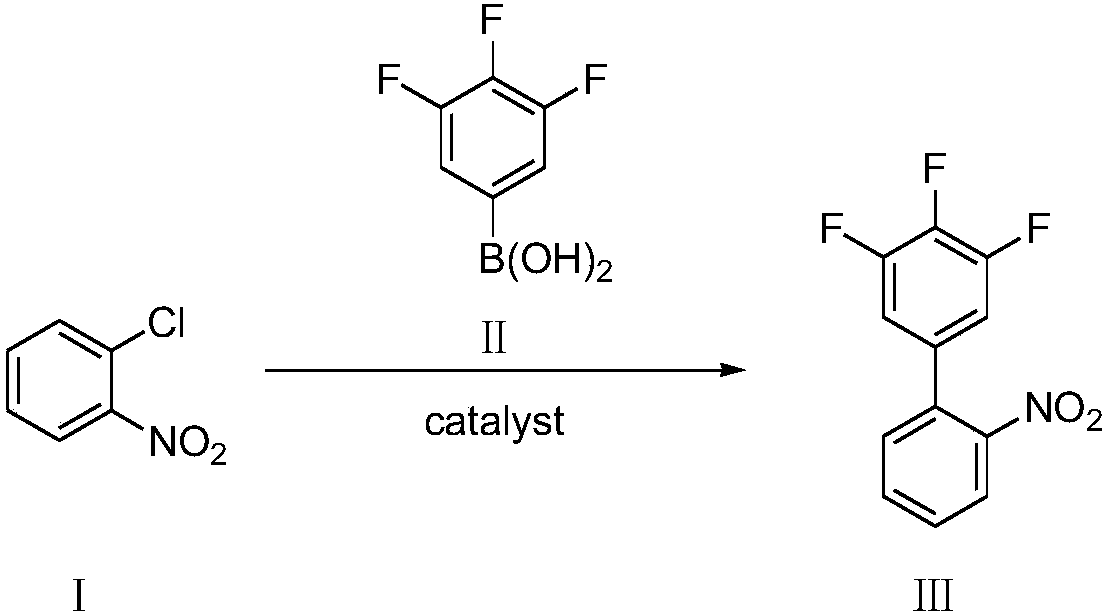
Preparation method of 4-bromocarbazole
ActiveCN103936656AImprove the coordination effectHigh yieldOrganic chemistryChemical synthesisPhosphorous acid
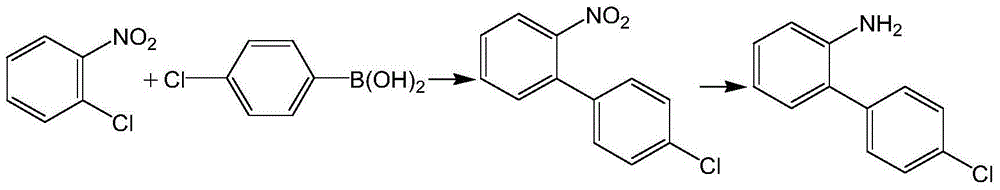
The invention discloses a preparation method of 4-bromocarbazole, which belongs to the field of organic chemical synthesis. The method comprises the following steps: by using o-bromophenylboronic acid as a raw material, a DMSO (dimethylsulfoxide) solvent and o-chloronitrobenzene to generate Suzuki reaction in the presence of combined catalysis of palladium metal and an organic phosphine ligand so as to obtain 2-bromine-2'-nitrobiphenyl; and then synthesizing a product 4-bromocarbazole, from 2-bromine-2'-nitrobiphenyl by using triphenyl phosphite as a reductant. During reaction, side reactions are few, the method is simple to operate and the yield is high. The synthesized 4-bromocarbazole can be used in the fields of organic photoelectric materials, medicines and the like, and is an important intermediate for carbazole photoelectric materials, medicines and presides.
Owner:PUYANG HUICHENG ELECTRONICS MATERIAL +1
Process of preparing 2,3,4-trifluoro nitrobenzene using o-chloro nitrobenzene
InactiveCN1357530AImprove general performanceReduce manufacturing costOrganic chemistryOrganic compound preparationHigh volume manufacturingDistillation
The preparation of 2,3,4-trifluoro nitrobenzene, the main intermediate for preparing Ofloxacin with o-chloronitrobenzene as raw material includes the steps of: chlorinizartion of o-chloronitrobenzene material, washing with washing liquid and decompressing rectification to prepare 2,3-dichloronitrobenzene; drying 2,3-dichloronitrobenzene and fluorination with KF in the presence of phase transfer catalyst; distillation to separate and to obtain fluorination product and chlorination and nitration at high temperature; and dewatering nitrified product, fluorination with KF and distillation to obtain 2,3,4-trifluoro nitrobenzene. The present invention reduces 2,3,4-trifluoro nitrobenzene producting cost greatly.
Owner:昆山虹祺药业有限责任公司
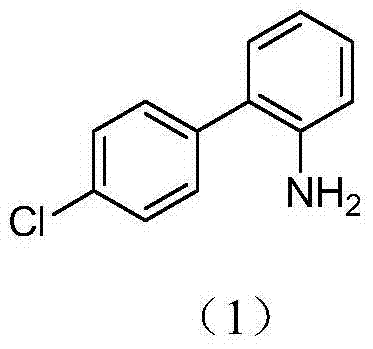
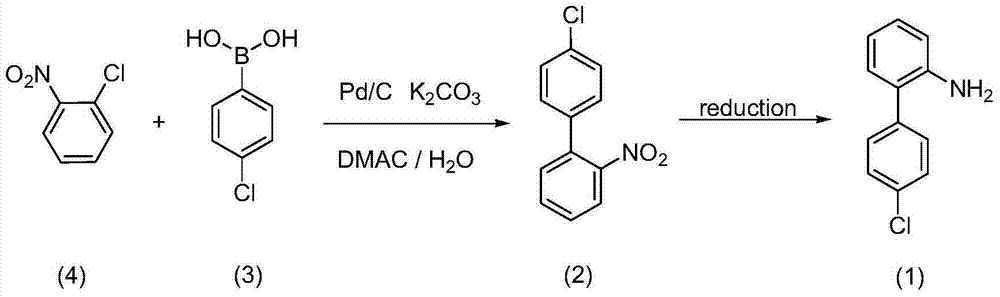
Method for preparing boscalid intermediate 2-(4-chlorophenyl) aniline
ActiveCN104529794AReduce dosageIncrease Cl activityOrganic compound preparationAmino compound preparationNitrobenzeneSolvent
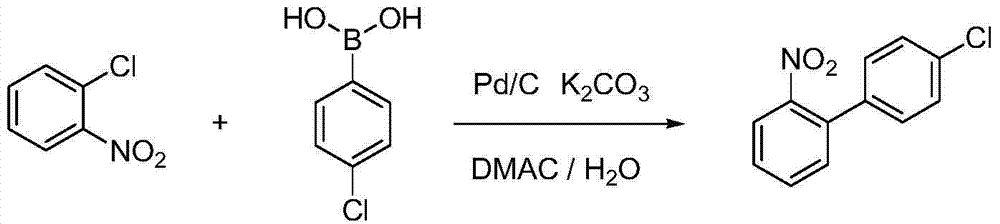
The invention belongs to the field of pesticides, relates to a technology for preparing agricultural fungicide intermediates and particularly relates to a technology for preparing boscalid intermediate 2-(4-chlorophenyl) aniline. The technology comprises the following steps of by adopting alcohol or formamide solvent, in an alkaline reagent and a KI system, under the action of Pd catalyst, carrying out Suzuki coupling reaction on o-chloronitrobenzene and p-chlorophenylboronic acid as raw materials to generate 2-(4-chlorophenyl)nitrobenzene, simply filtering the reaction system and directly carrying out catalytic hydrogenation on the reaction system to obtain the product. By the technology, the problems of high price of the raw materials, low yield, complex after-treatment and harsh reaction conditions of the traditional synthesis process of the boscalid intermediate are solved, the simple reaction is achieved in a true sense, the manufacturing cost is decreased, the content and yield of the product are increased and the technology is more conducive to industrial production.
Owner:JINGBO AGROCHEM TECH CO LTD
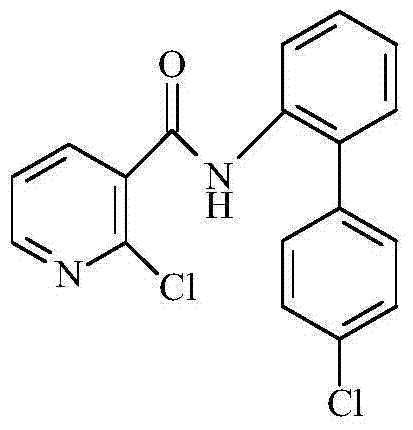
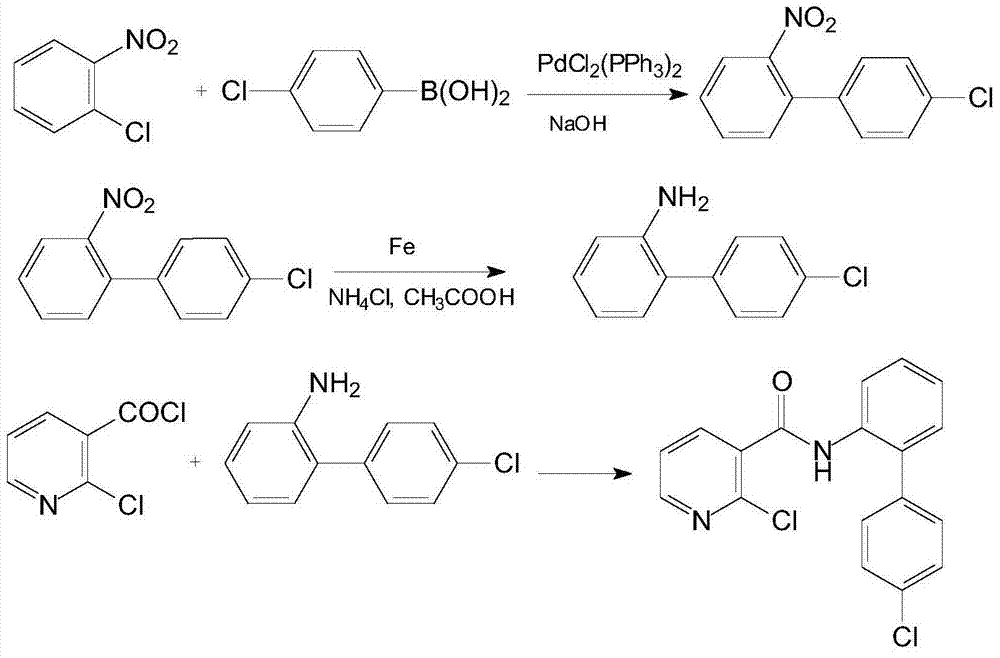
Synthetic method of 2-chloro-N-(4'-chlorodiphenyl-2-yl) nicotinamide
The invention discloses a synthetic method of 2-chloro-N-(4'-chlorodiphenyl-2-yl) nicotinamide. The method comprises the following steps: by taking o-chloronitrobenzene and 4-chlorobenzene boric acid as raw materials, performing Suzuki coupling reaction to generate a intermediate 4'-chloro-2-nitryldiphenyl; subsequently reducing to generate a intermediate 4'-chloro-2-aminodiphenyl; finally condensing the intermediate 4'-chloro-2-nitryldiphenyl and the intermediate 4'-chloro-2-aminodiphenyl with 2-chloronicotinoyl chloride to obtain a target product 2-chloro-N-(4'-chlorodiphenyl-2-yl) nicotinamide. The method is used for preparing the 2-chloro-N-(4'-chlorodiphenyl-2-yl) nicotinamide and has the advantages of mild reaction condition, simple process, low cost, high yield and the like.
Owner:XIAN MODERN CHEM RES INST
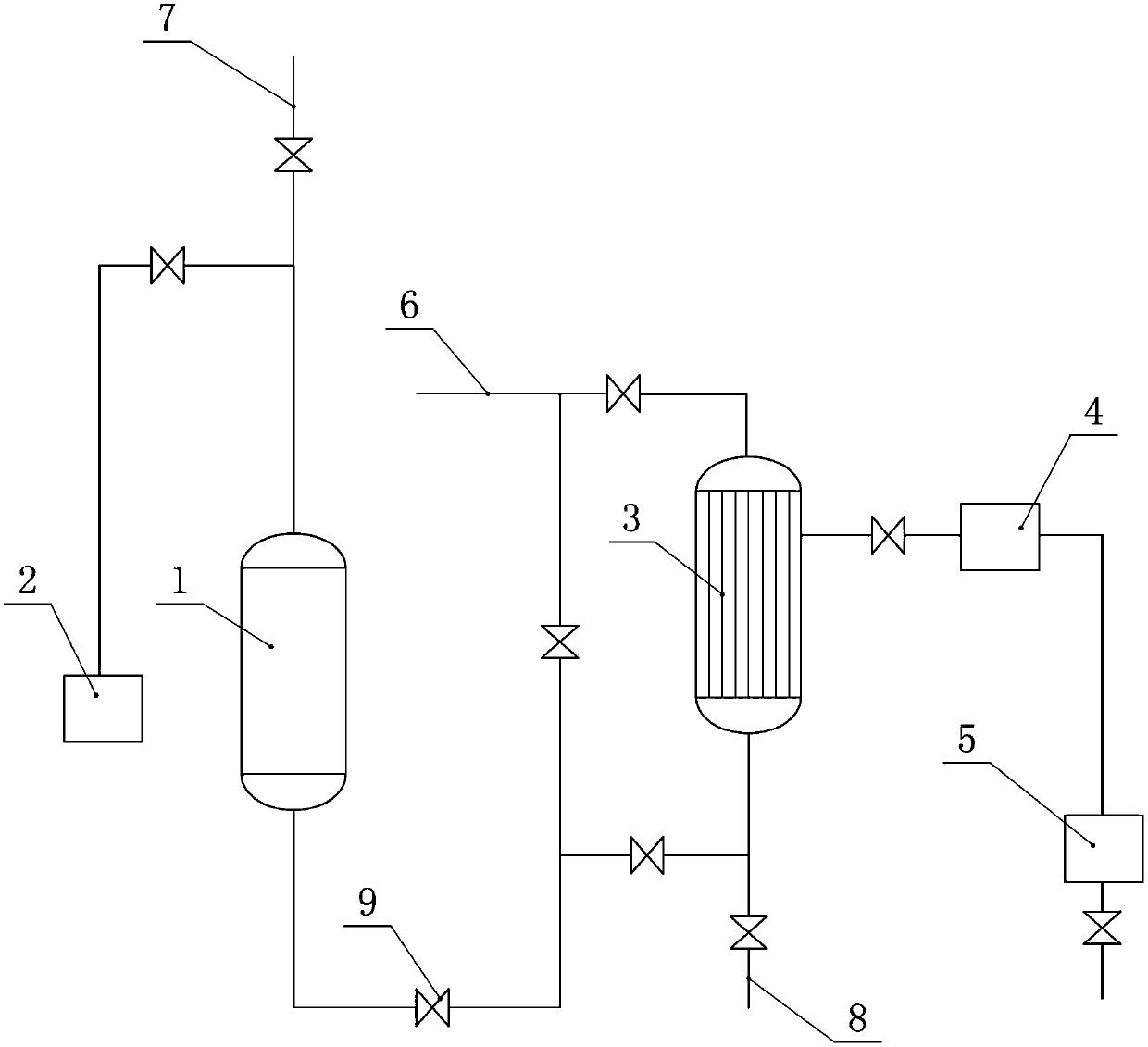
Method and device for producing o-chloroaniline without solvent
InactiveCN103387498AReduce labor intensityEasy to operateOrganic compound preparationChemical recyclingSolventPollution
The invention discloses a method and a device for producing o-chloroaniline without a solvent. The method utilizes o-chloronitrobenzene as a raw material, a dechlorination inhibitor as an assistant 1, ammonia water as an assistant 2 and a platinum-carbon catalyst. At a hydrogenation liquid pH of 6-8, under the pressure of 0.5-0.9Mpa, at a temperature of 85-105 DEG C, hydrogen is fed into a reactor and the reaction system undergoes a continuous reaction in the reactor; then the hydrogenation liquid is collected in a hydrogenation liquid tank by a catalyst filter; the catalyst filtered by the catalyst filter is blown back into the reactor by the catalyst filter for recycle; and the hydrogenation liquid subjected to water separation is fed into a rectification system and then is rectified so that a qualified product is obtained. The method and the device have the advantages of simple processes, low production cost, high automation degree, high yield, high output, high reaction selectivity, good product quality, mild reaction conditions, good safety, low labor intensity, no pollution, and easy popularization.
Owner:王一如
Method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction
ActiveCN101585771ATake advantage ofAvoid direct separationOrganic chemistryOrganic compound preparationChlorobenzenePotassium fluoride
The invention discloses a method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction, comprising steps of: generating a mixture mainly composed of parachloronitrobenzene, o-chloronitrobenzene and m-chloronitrobenzene in process of parachloronitrobenzene, o-chloronitrobenzene by chlorobenzene nitration method; removing low-boiling-point substances by evaporation; then adding anhydrous potassium fluoride and catalyst to the mixture for fluoro-reaction at 150 DEG. C to 250 DEG. C; after reaction, filtering to remove potassium chloride, after rectifying to acquire parachloronitrobenzene, o-chloronitrobenzene, the residual portion via recrystallization acquires m-chloronitrobenzene; the catalyst is quaternary ammonium salt or calixarene; charge rate of chloronitrobenzene mixture and mixture is 1:0.01 to 1. The method of the invention is simple to operate, in scale and changes waste material into things of value, which realizes zero discharge. The method is characterizedin sustainable development, energy saving, consumption reduction and environmentally friendly property.
Owner:浙江省常山长盛化工有限公司
Method for preparing 4'-chloro-2-aminobiphenyl through palladium/carbon catalysis
ActiveCN103539679ALow costSimple and fast operationOrganic compound preparationChemical recyclingIron powderReaction temperature
The invention relates to a method for preparing 4'-chloro-2-aminobiphenyl through palladium / carbon catalysis. The method comprises the steps: reacting in a solvent under a heating condition by taking p-chlorophenylboronic acid and o-chloronitrobenzene as raw materials, palladium / carbon as a catalyst and a weak base as a catalyst promoter to generate 4'-chloro-2-nitrobiphenyl; reducing 4'-chloro-2-nitrobiphenyl in a polar solvent by using a reducing agent zinc or iron powder under the heating condition to obtain 4'-chloro-2-aminobiphenyl. The invention finds a method which is lower in cost, simpler and more convenient to operate and environment-friendly through screening and researching yield influencing factors such as catalyst dosage, reaction solvent proportion, reaction temperature, reaction time, palladium / carbon recovered and reused times and the like in palladium / carbon catalytic coupling reaction.
Owner:天津均凯农业科技有限公司
Synthesis technique of benzotriazole
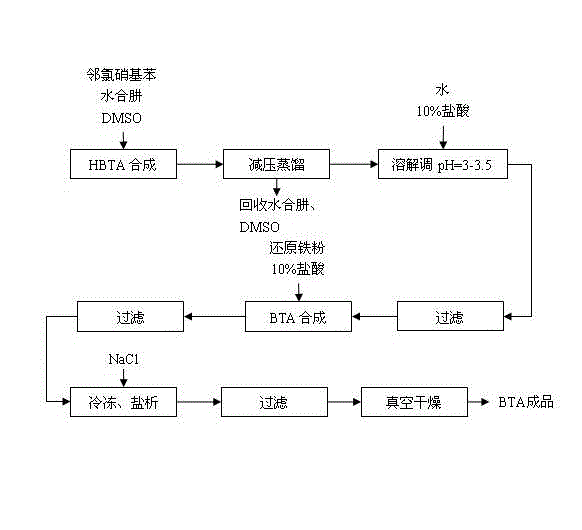
The invention relates to a synthesis technique of benzotriazole, which comprises the following two reaction steps: 1. reacting o-chloronitrobenzene and hydrazine hydrate to generate 1-hydroxybenzotriazole (HBTA), wherein DMSO (dimethyl sulfoxide) is used as a solvent to implement synthetic reaction of HBTA under homogeneous conditions; and 2. reacting the HBTA and reduced iron powder to generate the benzotriazole product. The DMSO with strong polarity promotes the reaction, so that the hydrazine hydrate can be added at one time, and the consumption of the hydrazine hydrate is reduced as compared with the existing technique; and meanwhile, the DMSO and hydrazine hydrate can be conveniently recovered. In the after-treatment process of preparing benzotriazole by reducing the HBTA, the salting-out mode is utilized to separate the product, thereby greatly enhancing the production efficiency and lowering the production cost as compared with the existing extraction method.
Owner:陈守文
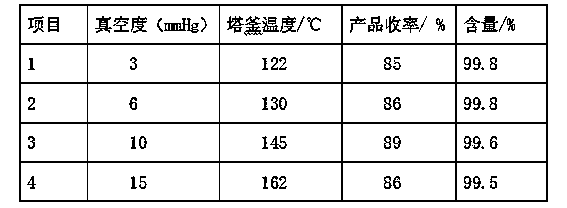
Purification process for o-phenylenediamine
ActiveCN103435495ASimple processImprove product qualityAmino compound purification/separationDistillationNitrobenzene
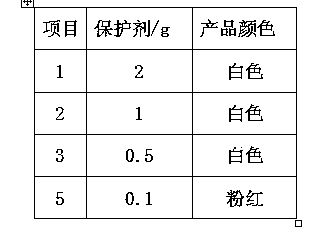
The invention discloses a purification process for o-phenylenediamine. An o-phenylenediamine crude product is used as a raw material and an ammonolysis and reduction process of o-chloronitrobenzene is employed by the synthesis of o-phenylenediamine. The purification process comprises the steps of transporting a crude product of o-phenylenediamine to a rectification tower kettle to carry out distillation and purification. During the whole rectification process, a vacuum degree is controlled at 6-10 mmHg; and a temperature of the tower kettle is controlled at about 145 + / -3 DEG C. Finally, a proper amount of a protective agent is added in materials of a product; and the materials are flaked under the vacuum or nitrogen protection to obtain a white o-phenylenediamine finished product. The purification process is advantageous in that process is simple; operation is stable and reliable; energy consumption is low; product quality is stable; and product yield is increased.
Owner:NANTONG BOTAO CHEM
Method for preparing 4'-chloro-2-nitrobiphenyl
InactiveCN105732392AEasy to buyReduce pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPalladium catalystPotassium
The invention provides a method for preparing 4'-chloro-2-nitrobiphenyl. The method comprises the following steps: o-chloronitrobenzene and p-chloro potassium benzyltrifluoroborate are adopted as raw materials which are added with a phase transfer catalyst and a palladium catalyst; and the 4'-chloro-2-nitrobiphenyl is obtained through reactions in an aqueous solution under a heating condition, wherein the reactions are carried out under a weak-base condition. The preparation method provided by the invention has the advantages of simplicity in operation, easily available raw materials, little environmental pollution, high yield, relatively good product quality and the like.
Owner:天津均凯农业科技有限公司
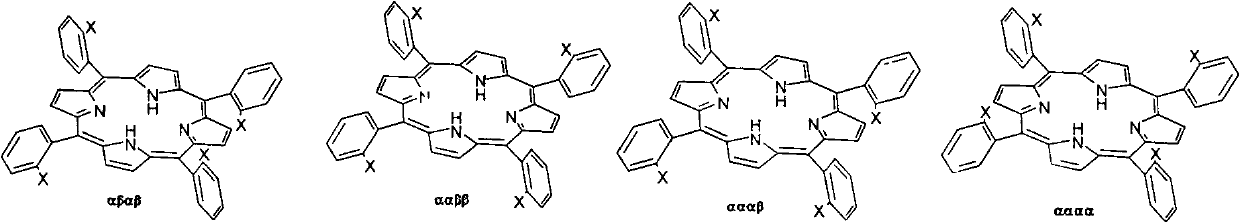
Preparation method for tetra-(o-pivaloyl amino phenyl) zinc protoporphyrin isomer compound
InactiveCN102391270AHigh yieldThe synthesis method is simpleOrganic chemistryPivalamideReaction temperature
The invention relates to a preparation method for tetra-(o-pivaloyl amino phenyl) zinc protoporphyrin isomer compound, which comprises the following steps: by taking tetra-o-chloronitrobenzene protoporphyrin as a raw material and utilizing a three-step method, controlling the use amount of the raw material, reaction temperature and solvent selection to respectively synthesize four tetra-(o-pivaloyl amino phenyl) zinc protoporphyrin isomer compounds with extra high purity, wherein the tetra-(o-Pivaloyl amino phenyl) zinc protoporphyrin isomer compounds have the formula disclosed in (I). According to the preparation method, the purification process of the metal protoporphyrin compound containing the isomer is greatly simplified, and the preparation method is simple to operate and has the advantages of available raw material and high yield.
Owner:EAST CHINA UNIV OF SCI & TECH
2,2'-dichlorohydrazobenzene preparation method and special electrolytic tank thereof
ActiveCN101597765AImprove mechanical propertiesAvoid polarizationCellsElectrolytic organic productionElectricityElectrolysis
The invention discloses a 2,2'-dichlorohydrazobenzene preparation method and a special electrolytic tank thereof; the preparation method comprises the following steps: separately placing cathode alkali liquor and anode alkali liquor in the cathode tank and the anode tank of the electrolytic tank, preheating the cathode alkali liquor in the cathode tank at 30-60 DEG C for 15-35min while introducing an electric current activation porous negative plate 5 and a porous positive plate 4 with a current density of 1-2.5A / dm2; then adding the reactants containing o-chloronitrobenzene, toluene and promoter into the cathode tank 1 in turn, mixing and stirring the mixture evenly, and connecting a power supply with a voltage of 1.42-2.65V, an electrolytic current density of 1-5A and an electrolytic temperature of 50-80 DEG C. The preparation method of the invention has the advantages of simple process, mild technology condition, low electricity consumption and low cost.
Owner:HANERGY TECH
Electrochemical method for synthesizing 2,2'-dichlorohydrazobenzene by use of supported catalyst ionic membrane
The invention relates to an electrochemical method for synthesizing 2,2'-dichlorohydrazobenzene by use of a supported catalyst ionic membrane. The method is used for preparing a catalyst which is supported on an ionic exchange membrane, so that the membrane has a higher hydrogen generation rate, the ionic exchange capacity is increased, and the electrochemical performance of the ionic exchange membrane is optimized. The ionic exchange membrane is applied to a reaction of electrochemical synthesis of 2,2'-dichlorohydrazobenzene from o-chloronitrobenzene; an electrolytic tank is separated into a cathode chamber and an anode chamber by the supported catalyst ionic exchange membrane; a sodium hydroxide solution is added into the cathode chamber and the anode chamber; the raw material o-chloronitrobenzene generates a reduction reaction in the cathode chamber; the supported catalyst ionic membrane realizes a great effect on promoting the increase of the hydrogen generation rate in the reducing process, and the reaction efficiency is improved; and the method provided by the invention has the characteristics of short technological process and high product yield.
Owner:BEIJING UNIV OF CHEM TECH
Synthesis process of boscalid intermediate 2-(4-chlorphenyl)phenylamine
InactiveCN106748804AReduce pollutionGood workmanshipOrganic compound preparationGroup 3/13 element organic compoundsSuzuki reactionCoupling
The invention discloses a synthesis process of boscalid intermediate 2-(4-chlorphenyl)phenylamine. Chlorophenylboronic acid and o-chloronitrobenzene are taken as starting raw materials, and a main reaction consisting of the two steps of Suziki and hydrogenation reduction is carried out to prepare the 2-(4-chlorphenyl)phenylamine. 1, optimal reaction steps determined through continuous testing are adopted, a proper solvent is selected preferably, and the 2-(4-chlorphenyl)phenylamine is prepared at a proper temperature and under a proper pressure, so that a superior process, high yield and high working efficiency are realized, no noble metal coupling agent is needed, the environmental pollution is lowered, raw materials are readily available, and the production cost is lowered; 2, a target product is obtained by obtaining a solid through concentration and cooling, so that a posttreatment method is simplified effectively, and meanwhile the content and high yield of the product are ensured effectively; 3, the Suzuki reaction steps are simple, filtrate left after the reaction can be directly applied to a next-step hydrogenation reduction reaction, operation is easy, no pollutant waste is produced in the hydrogenation reduction reaction, and the environment and the health of operating personnel are protected.
Owner:ZHEJIANG RONGKAI TECH DEV
Prepn process of 2,4-dichlorofluorobenzene
InactiveCN1357524AReduce the amount of investmentLow costHalogenated hydrocarbon preparationPotassium fluorideChloride
The present invention prepares 2,4-dichlorofluorobenzene by using o-chloronitrobenzene as raw material and through the steps of: electrophilic substitution reaction between o-chloronitrobenzene and chlorine gas on benzene ring in the presence of catalyst; the reaction between the refined product of the fore reaction and potassium fluoride, filtering and washing to obtain 2-fluoro-5-chloronitrobenzene; chlorination of 2-fluoro-5-chloronitrobenzene at high temperature to obtain the product 2,4-dichlorofluorobenzene. The present invention has low material cost and no explosion danger.
Owner:昆山虹祺药业有限责任公司
Preparation of a nitrogen-doped carbon-coated core-shell nickel-iron alloy nanocatalyst and its application in the hydrogenation of o-chloronitrobenzene
ActiveCN106732733BMaterial nanotechnologyPhysical/chemical process catalystsNitro compoundNano catalyst
The invention provides a preparation method of a nitrogen-doped carbon-coated core-shell structure nickel-iron alloy nanocatalyst in the field of catalyst technology and its application in catalyzing the hydrogenation reaction of o-chloronitrobenzene. The method first synthesizes a nickel-iron layered double metal hydroxide precursor with small particle size and high surface energy through a nucleation and crystallization isolation method, and then uniformly mixes it with a melamine and dicyandiamide mixed carbon material precursor. , and finally a new type of nitrogen-doped carbon-coated core-shell structure nickel-iron alloy nanocatalyst was prepared through high-temperature self-reduction. It is efficiently used in the catalytic hydrogenation of halogenated nitro compounds to produce halogenated anilines. The conversion rate of o-chloronitrobenzene and the selectivity of o-chloroaniline can reach 95-100% and 98-100% respectively. . This new nitrogen-doped carbon-coated core-shell structure nickel-iron alloy nanocatalyst has a novel and unique structure, a green and energy-saving process, and a stable catalyst structure, and has broad application prospects.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of 3,4,5-trifluoro-2'-nitrobiphenyl
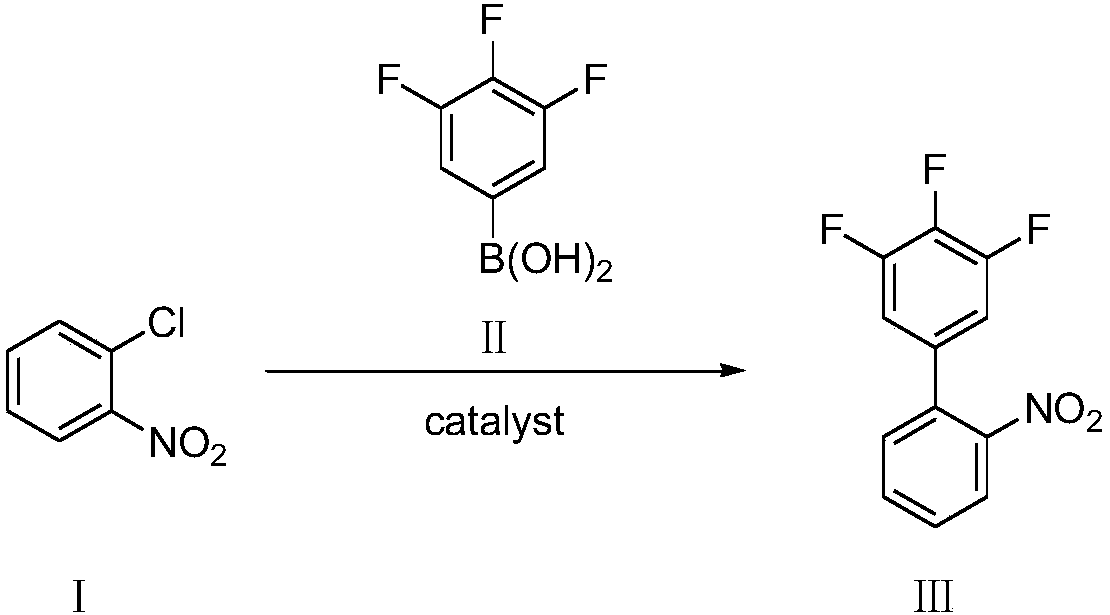
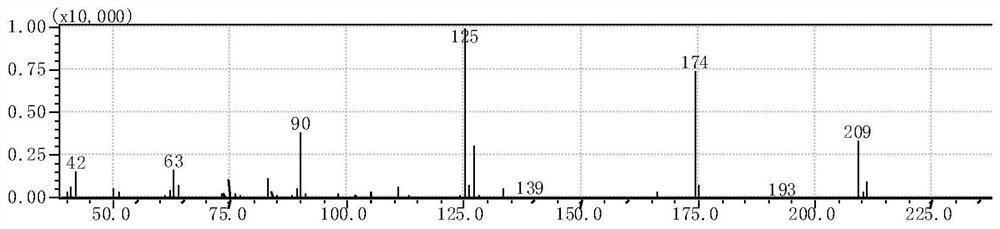
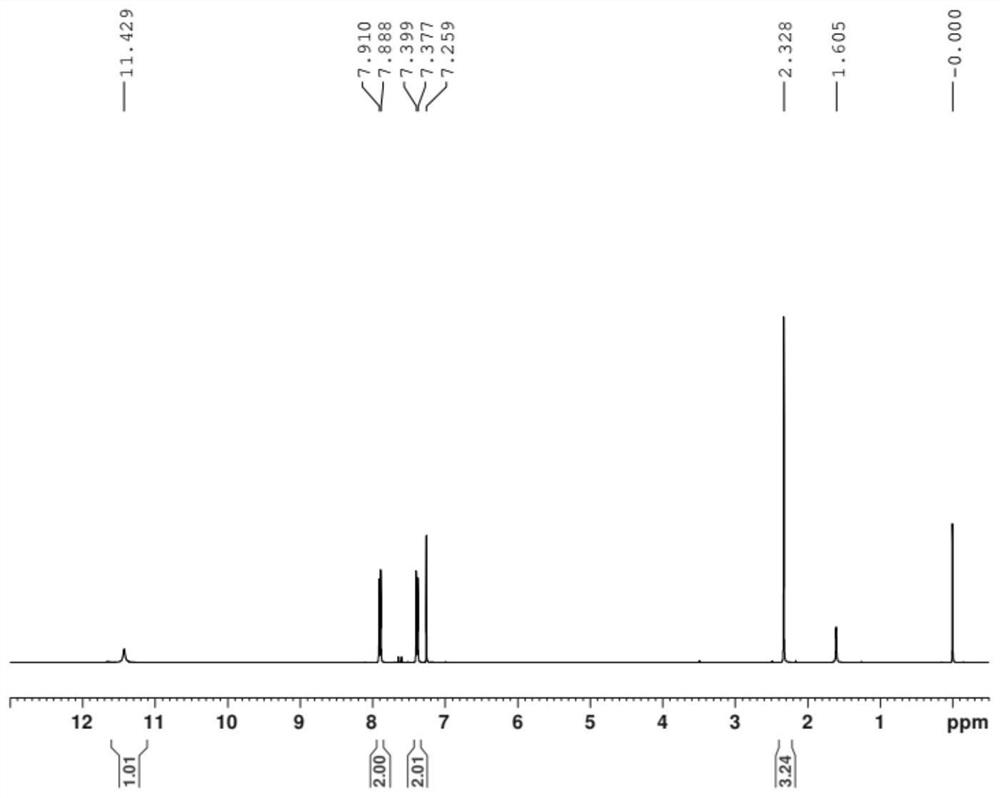
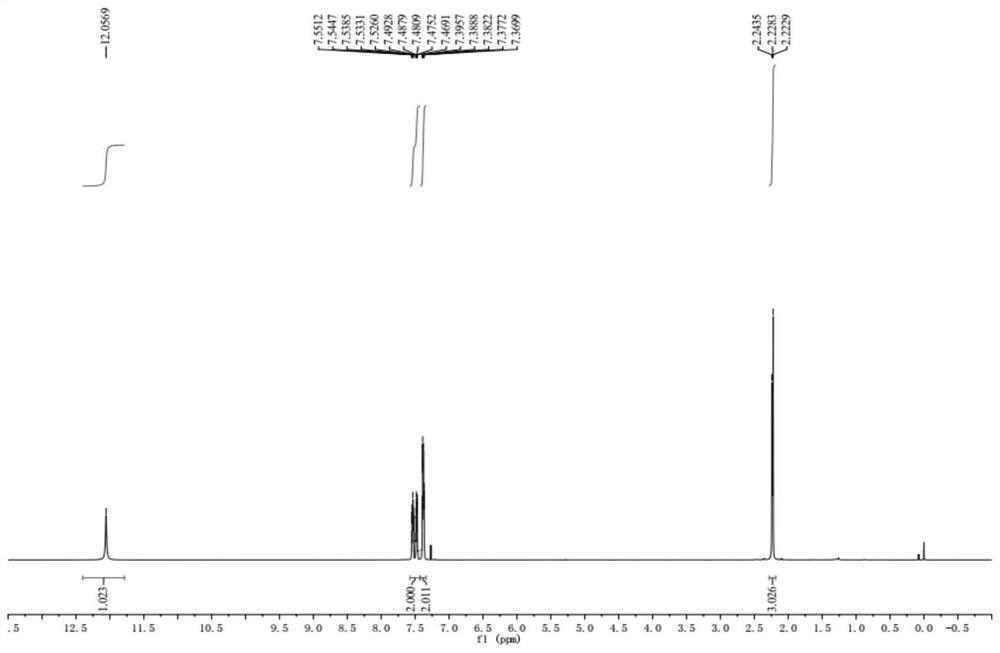
ActiveCN109956871AEfficient preparationAftertreatment is simple and environmentally friendlyOrganic chemistryOrganic compound preparationPhase-transfer catalystO-chloronitrobenzene
The invention discloses a preparation method of 3,4,5-trifluoro-2'-nitrobiphenyl. The method comprises the following steps: water is used as a solvent, o-chloronitrobenzene and 3,4,5-trifluorobenzeneboronic acid are subjected to a reaction in the presence of a phase transfer catalyst under the action of an acid binding agent, a catalyst and a ligand of the catalyst, and therefore the 3,4,5-trifluoro-2'-nitrobiphenyl is obtained. The method provided by the invention adopts pure water phase reaction, and has the advantages of greenness, environmental protection and convenient post-treatment.
Owner:ZHEJIANG RES INST OF CHEM IND CO LTD +1
Synthesis method of sulfentrazone intermediate
ActiveCN113402472AReduce manufacturing costAvoid supply constraintsOrganic chemistryP-chloroanilineChlorobenzene
The present invention provides a synthesis method of asulfentrazone intermediate. The methodcomprises: S1) carrying out a nitration reaction on chlorobenzene in a nitration reagent to obtain a mixture of o-chloronitrobenzene and p-chloronitrobenzene, wherein the product does not need to be separated; s2) performing catalytic hydrogenation reaction on the mixture of o-chloronitrobenzene and p-chloronitrobenzene to obtain a mixture of o-chloroaniline and p-chloroaniline, wherein the product does not need to be separated; s3) making the mixture of o-chloroaniline and p-chloroaniline subjected to a diazotization reaction to obtain a mixture of o-chlorophenylhydrazine and p-chlorophenylhydrazine, wherein the product does not need to be separated; s4) performing condensation reaction on the mixture of the o-chlorophenylhydrazine and the p-chlorophenylhydrazine and aldehyde to obtain triazole ring mixtures as shown in a formula I-a and a formula I-b; and S5) carrying out chlorination reaction on the triazole ring mixtures to obtain the sulfentrazone intermediate shown in the formula I. According to the method, 2, 4-dichloroaniline is not used as a raw material, so that the production cost of sulfentrazone is reduced, and the limitation of raw material supply is avoided.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Synthesis method of o-amino pheylmethyl ether
InactiveCN104086448AEasy to useEasy to prepareOrganic compound preparationAmino-hyroxy compound preparationChemical industryO-nitrochlorobenzene
The invention discloses a preparation method of o-amino pheylmethyl ether, and relates to the technical field of chemical industry. The method comprises the following steps: adding o-chloronitrobenzene, methanol and a 40-percent sodium hydroxide solution into a high-pressure reaction kettle in sequence, raising the temperature in the kettle to 40 DEG C, and stirring; raising the temperature to 85 DEG C, controlling the pressure at 0.28-0.32MPa, reacting for 8 hours, distilling, removing an internal methanol solution, adding hot water of 70 DEG C for washing, standing for delaminating, and performing liquid separation to obtain o-nitroanisole for later use; putting o-nitroanisole into the high-pressure reaction kettle, adding a sodium sulfide aqueous solution, controlling the pressure at 0.05MPa, controlling the temperature at 118-120 DEG C, pressurizing, refluxing, cooling to 50-60 DEG C, preserving heat for 5 hours, performing liquid separation, removing internal waste water, distilling, crystalizing, drying to obtain finished o-amino pheylmethyl ether, packaging and warehousing. The preparation method has the beneficial effects of convenience and easiness in preparation, environmental friendliness, pollution freeness, ready availability of raw materials, small equipment investment, high purity and convenience in operation. The prepared o-amino pheylmethyl ether has a good use effect, and is safe and reliable.
Owner:安徽佑骏商品混凝土有限公司
Method for synthesizing N-o-nitrophenyl amino acid from o-chloronitrobenzene
InactiveCN102516103AOrganic compound preparationAmino-carboxyl compound preparationIodideNitrobenzene
The invention provides a method for synthesizing an N-o-nitrophenyl amino acid from o-chloronitrobenzene. Under the protection of argon and under the existence of cuprous iodide and potassium carbonate, the o-chloronitrobenzene reacts with amino acid, thereby obtaining the N-o-nitrophenyl amino acid. The method has the advantages of easiness in raw material obtaining, low cost and certain practical value.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Production process of catalytically hydrogenating nitrobenzene halide to synthesize haloarylamine
InactiveCN1199935CInhibition of hydrodehalogenationHigh catalytic activityOrganic compound preparationAmino compound preparationCarbon nanotubeNitrobenzene
The invention provides a production method for synthesizing halogenated aniline by catalytic hydrogenation of halogenated nitrobenzene. The production method uses carbon nanotubes to support Pd, Pt is a hydrogenation catalyst, p-halogenated nitrobenzene such as o-chloronitrobenzene, p-chloronitrobenzene, 3,4-dichloronitrobenzene, 3-chloro-4 The corresponding halogenated anilines were synthesized by liquid-phase catalytic hydrogenation reaction of -fluoronitrobenzene, etc. The production method can effectively suppress hydrodehalogenation, and has the advantages of good selectivity, high yield, high catalyst stability, etc., and the method has great implementation value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH
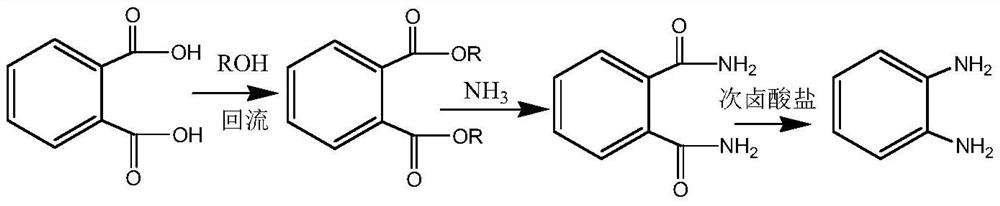
Preparation method of o-phenylenediamine
ActiveCN112812019AAvoid it happening againEmission reductionOrganic compound preparationCarboxylic acid esters preparationAlcoholNitrobenzene
The invention relates to a preparation method of o-phenylenediamine. The preparation method comprises the following steps: esterifying phthalic acid with alcohol under the catalysis of acid to prepare phthalate, then introducing ammonia gas into an alcohol solvent to obtain phthalic diamide, and then reacting the phthalic diamide with hypohalite or halogen in an alkaline solution to prepare the o-phenylenediamine. Compared with the prior art, the method disclosed by the invention completely avoids a mixed acid nitration process, does not generate waste acid, also does not cause safety accidents during ammonolysis of o-chloronitrobenzene, and is safe, clean and efficient.
Owner:上海呼龙科技开发有限公司
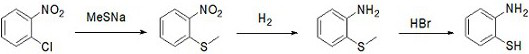
A kind of preparation method of o-aminothiophenol
ActiveCN112062699BHigh yieldHigh purityThiol preparationSulfide preparationPtru catalystNitrobenzene
The invention provides a preparation method of o-aminothiophenol. The preparation method comprises the following steps: putting o-chloronitrobenzene into sodium methyl mercaptide, and performing heating under the action of a catalyst to carry out a methyl vulcanization reaction so as to prepare o-nitrophenyl dimethyl sulfide; putting o-nitrophenyl thioether into a solvent, and carrying out hydrogenation reduction to prepare o-aminobenzene thioether; and demethylating the o-aminothiophenol under the action of hydrobromic acid to obtain the o-aminothiophenol. The preparation method of o-aminothiophenol has the advantages of high yield and high product purity.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
A kind of stainless steel mesh supported platinum catalyst and its application
ActiveCN103769164BHigh activityImprove stabilityOrganic compound preparationAmino compound preparationHydrogen atmosphereRoom temperature
Owner:ZHEJIANG UNIV OF TECH
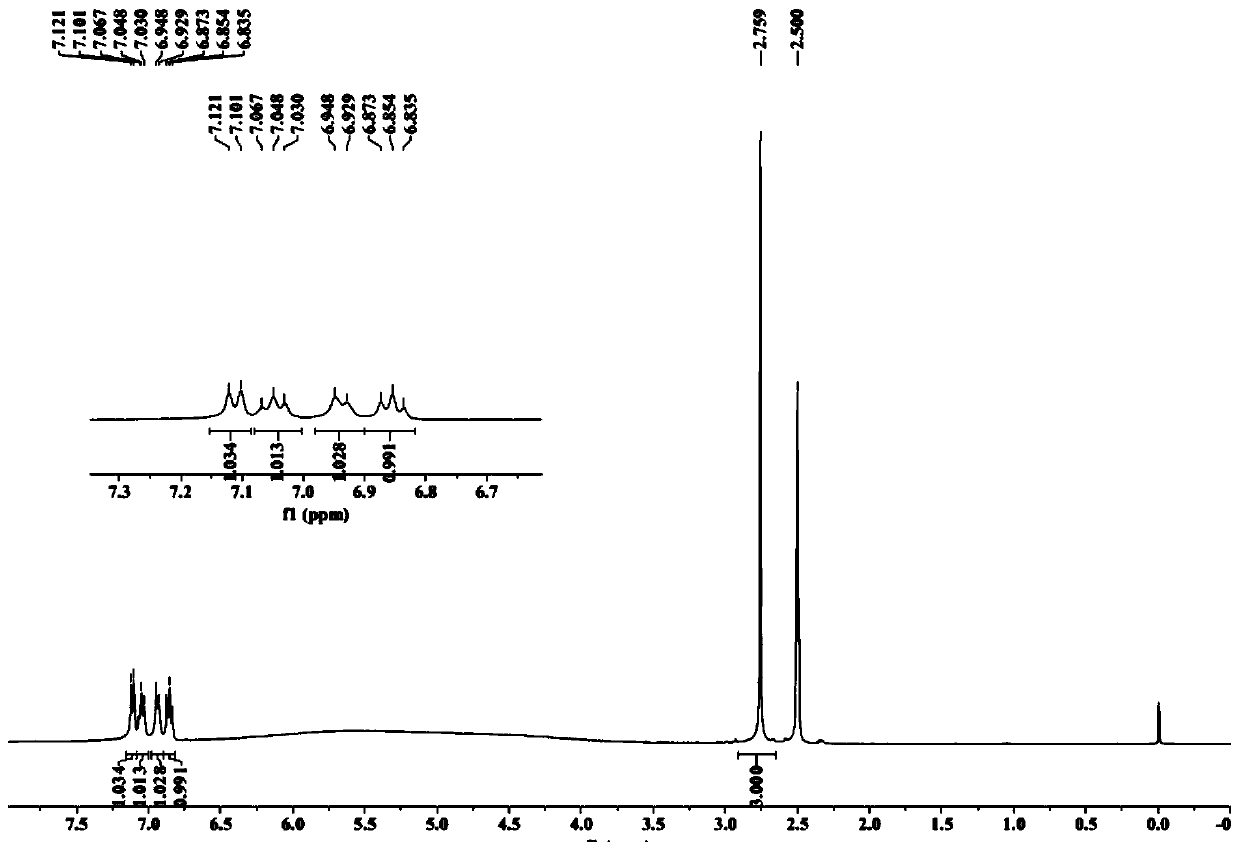
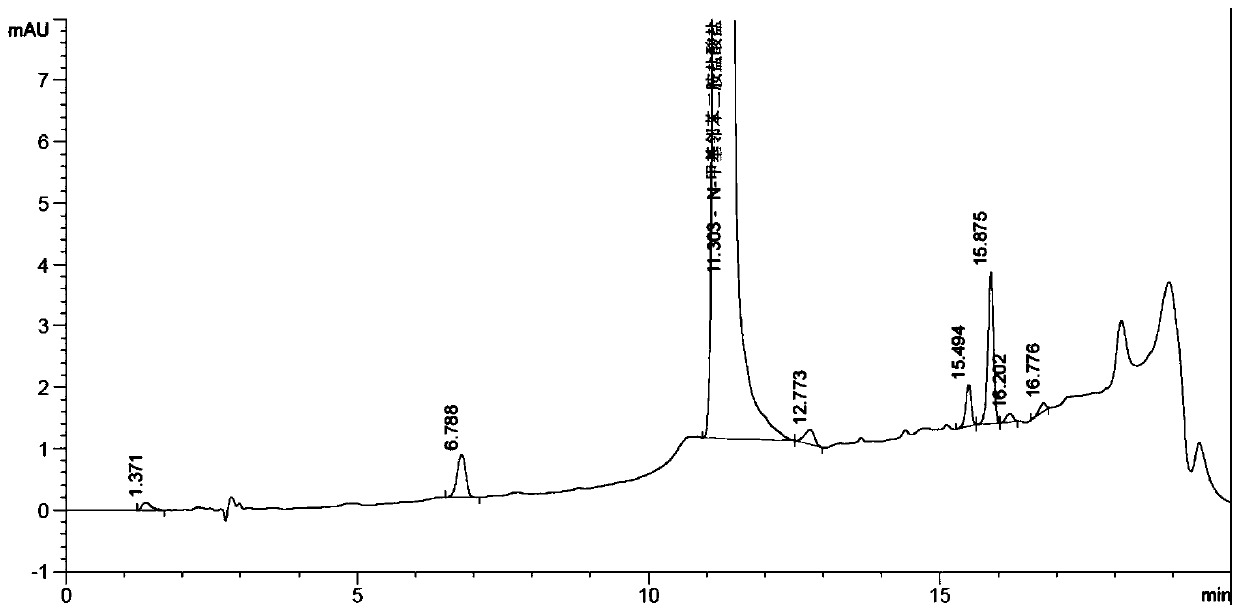
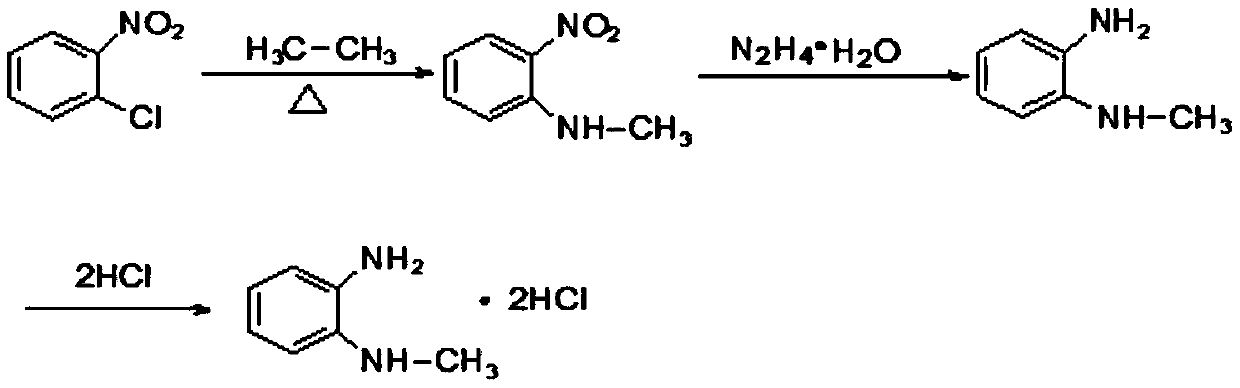
Synthesis method of N-methyl-1,2-benzenediamine dihydrochloride
InactiveCN110272347AHigh purityLess impuritiesOrganic compound preparationAmino compound preparationFiltrationSynthesis methods
The invention discloses a synthesis method of N-methyl-1,2-benzenediamine dihydrochloride. The synthesis method is characterized by comprising the following steps that 1, o-chloronitrobenzene and a monomethylamine aqueous solution are subjected to a sealing reaction, after the reaction is completed, cooling, standing and layering are conducted, and a lower-layer oil phase is collected to obtain N-methyl-2-nitroaniline; 2, a catalyst is added to a mixed solution of ethyl alcohol and the N-methyl-2-nitroaniline obtained in step 1, after mixing and heating, hydrazine hydrate is slowly dropwise added to the mixed solution, and after drop addition is completed, a heat preservation reaction is conducted; after the reaction is completed, suction filtration is conducted, and a suction filtration mother solution is reserved to obtain N-methyl-o-phenylenediamine; 3, liquid caustic soda, water and EDTA are added to the N-methyl-o-phenylenediamine obtained in step 2 for mixing, after cooling is conducted, dichloromethane is added, stirring, extraction, standing and layering are conducted, a lower-layer oil phase is collected, and through separation and purification, the target product N-methyl-1,2-benzenediamine dihydrochloride is obtained. The N-methyl-1,2-benzenediamine dihydrochloride product prepared through the method has high purity and few impurities and can be widely applied to the field of intermediate synthesis of a medicine tishamitan for reducing the blood pressure.
Owner:武汉本杰明医药股份有限公司
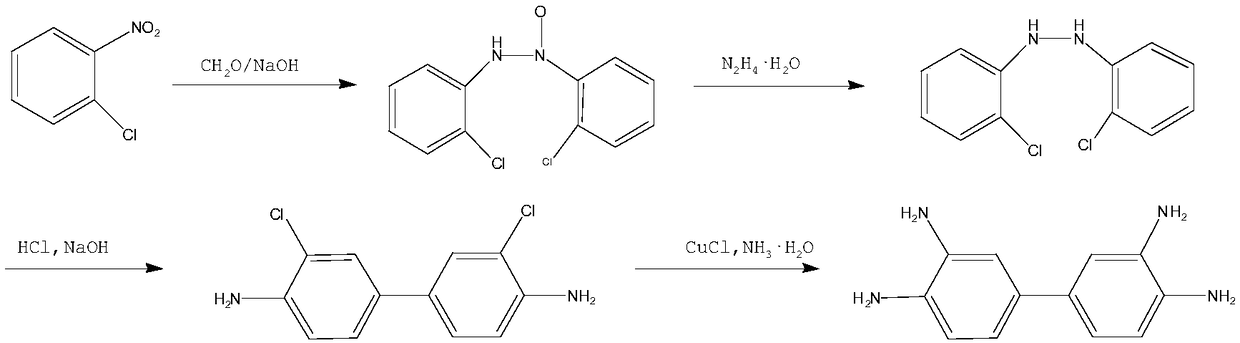
Preparation method of 3,3',4,4'-tetraaminobibenzene
ActiveCN108218711AReduce pollutionSimple processHydrazine preparationOrganic compound preparationFiltrationAlloy
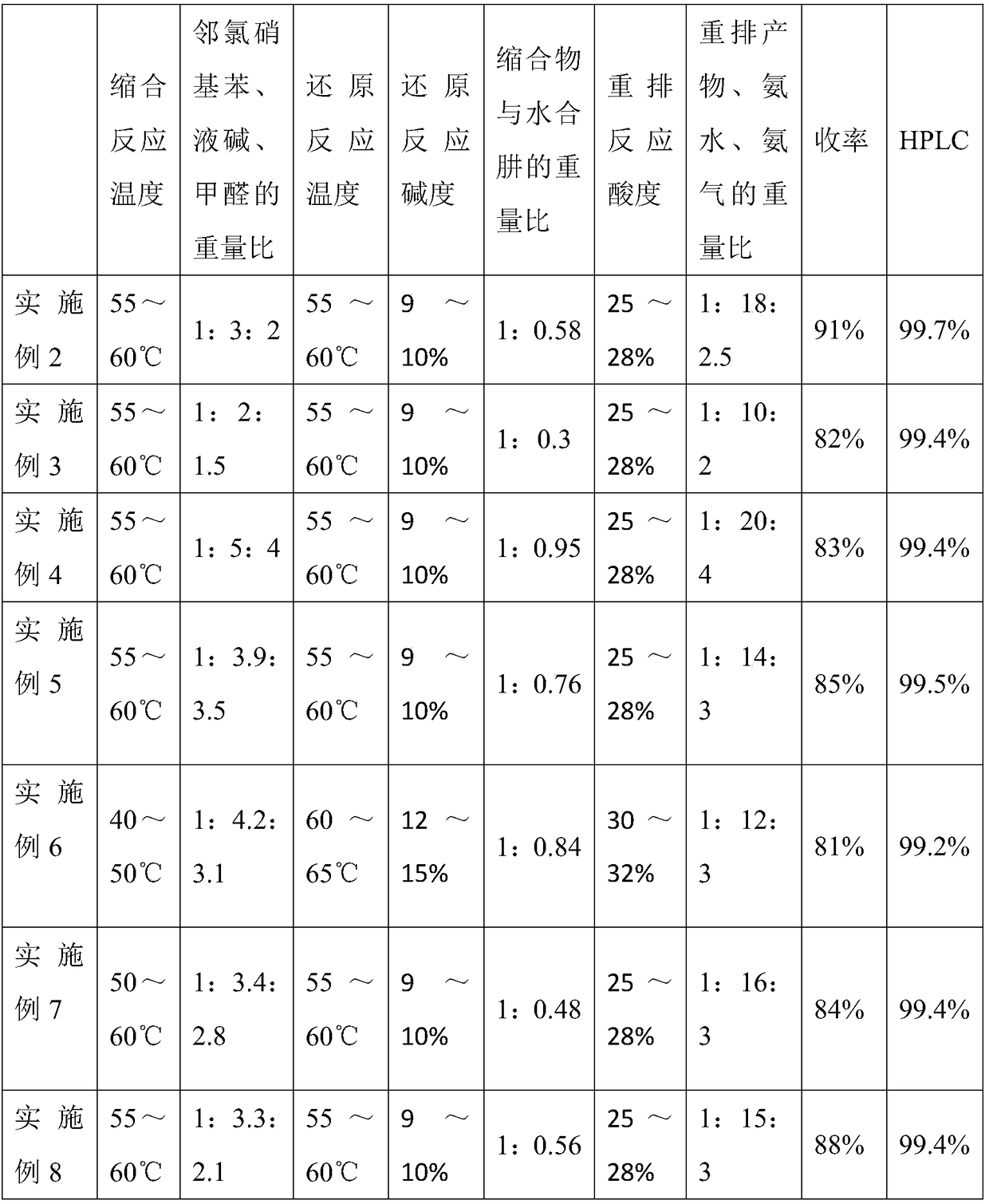
The invention relates to a preparation method of 3,3',4,4'-tetraaminobibenzene, which includes the steps of: (1) with an o-chloronitrobenzene compound, alkali, and an aldehyde compound as raw materials, performing a condensation reaction in a certain solvent with a 1,4-naphthoquinone compound as a catalyst; (2) adding the 1,4-naphthoquinone compound and Al / Ni alloy, as a reduction catalyst, to thecondensation product, and adding a sodium dodecyl benzene sulfonate compound as an emulsifier to perform a reaction with hydrazine hydrate to obtain a hydrogenated reaction solution, and performing temperature-reduced crystallization and re-crystallization, then performing temperature-reduced filtration or skimming concentration to obtain a hydrogenated product; (3) performing rearrangement reaction to the hydrogenated product under acidic conditions to obtain a rearrangement product; (4) with a copper salt compound as a catalyst, performing a reaction to the rearrangement product with ammonia water and ammonia gas at high temperature under high pressure, and cooling and filtering the product to obtain the 3,3',4,4'-tetraaminobibenzene. The method can improve quality of the product and reduce environment friendly, achieves cleanness and environmental protection of production and is suitable for large-scale production.
Owner:ZHEJIANG DINGLONG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com