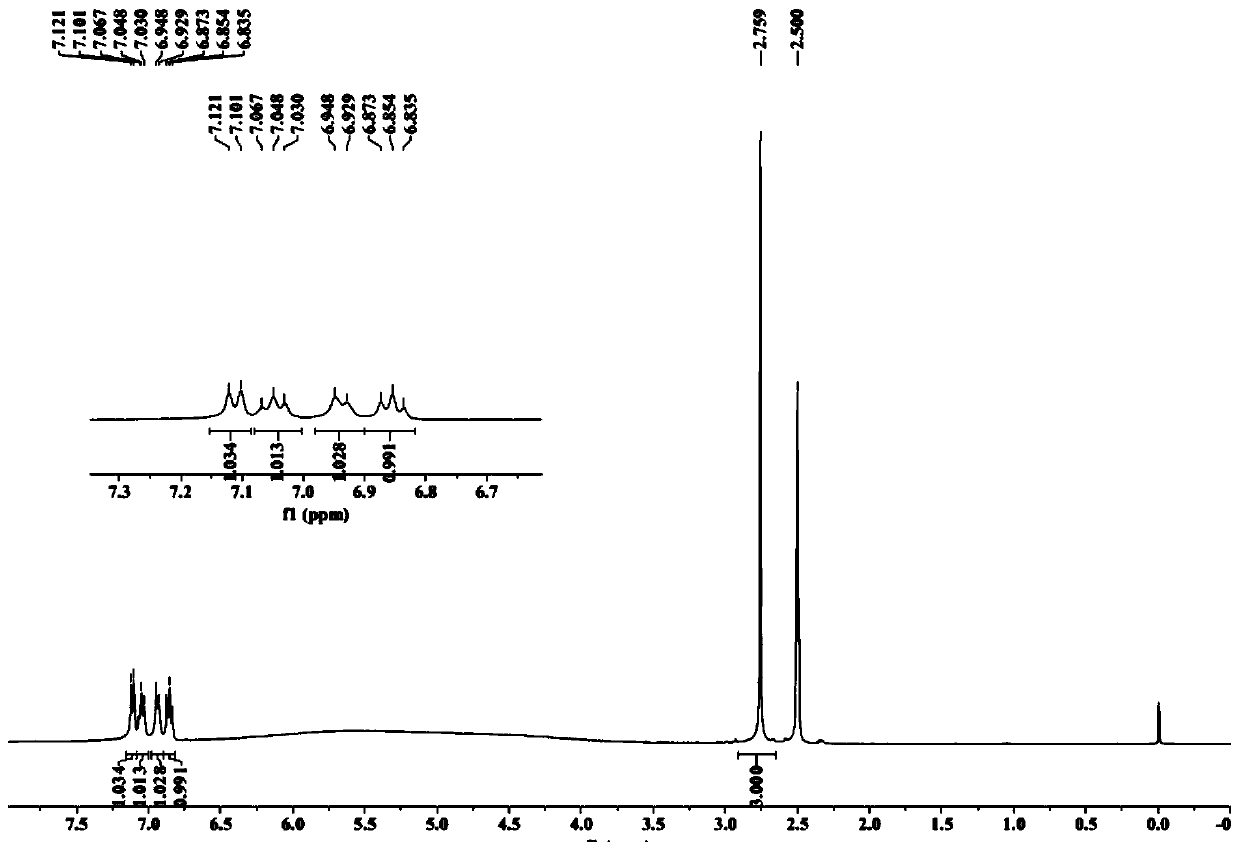
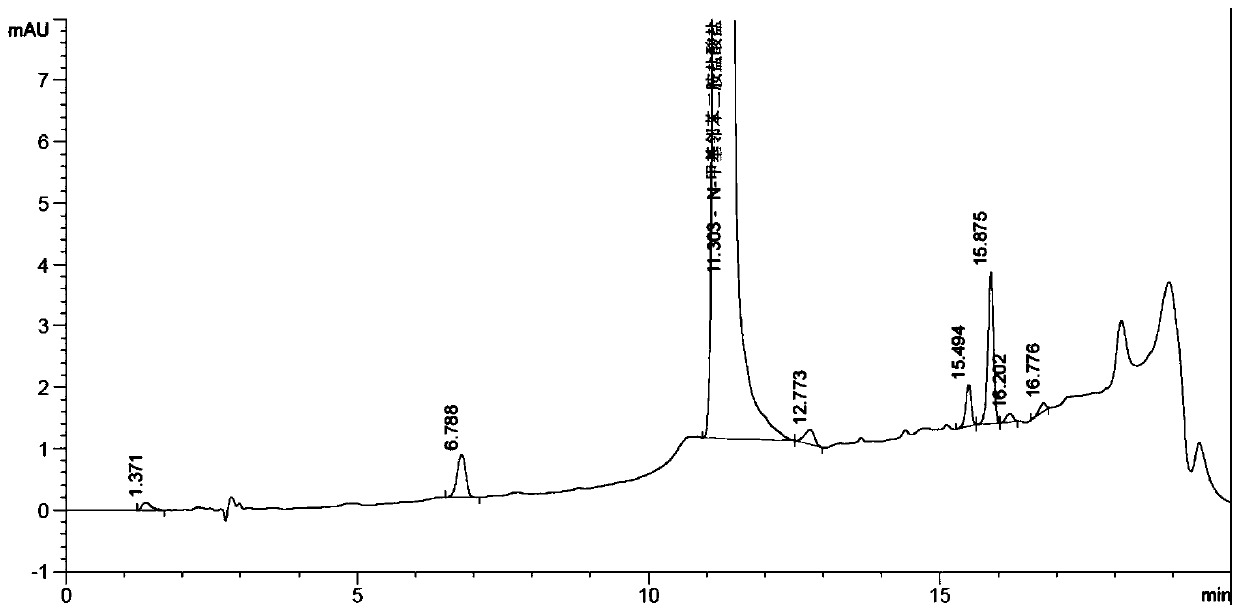
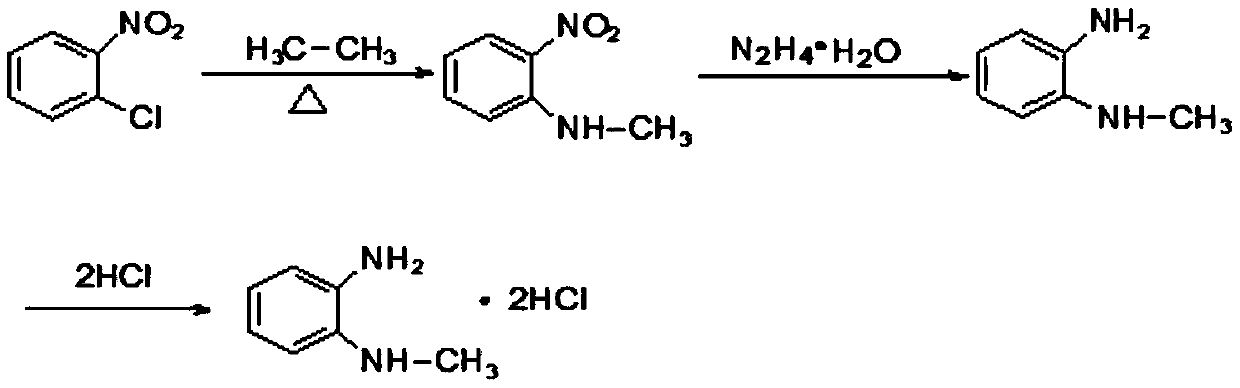
Synthesis method of N-methyl-1,2-benzenediamine dihydrochloride
A technology of methyl-o-phenylenediamine hydrochloride and methyl-o-phenylenediamine, which is applied in the field of synthesizing intermediates of the antihypertensive drug tesamitan, can solve the problems of difficult product quality assurance, low purity of o-diphenylamine, Problems such as low utilization rate, to achieve the effect of improving utilization rate, less impurities, and high preparation purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 200g of o-chloronitrobenzene and 197g of monomethylamine aqueous solution into a 1L autoclave, seal it, pressurize it with nitrogen to 0.5MPa, start to raise the temperature slowly, when T=90°C, stop heating, the reaction enters a spontaneous state, exotherm , the temperature continues to rise (the highest temperature can reach 110°C, and the maximum pressure is 1.2MPa). When the temperature no longer rises, continue to raise the temperature to 120°C, and keep the temperature for 5h. After the reaction was finished, the temperature was lowered, the layers were separated, and the lower oil phase was taken to obtain 190 g of N-methyl-o-nitroaniline.
[0034] Add 120g of ethanol and 100g of N-methyl-o-nitroaniline to a 1L reaction flask in turn, start stirring, raise the temperature to 70-75°C, keep a slight reflux, slowly add hydrazine hydrate dropwise, after the addition is complete, keep it warm at 80°C Reaction 20h. After the reaction was finished, filter with suc...
Embodiment 2
[0037] Add 200g of o-chloronitrobenzene and 197g of monomethylamine aqueous solution into a 1L autoclave, seal it, pressurize it with nitrogen to 0.5MPa, start to raise the temperature slowly, when T=90°C, stop heating, the reaction enters a spontaneous state, exotherm , the temperature continues to rise (the highest temperature can reach 110°C, and the maximum pressure is 1.2MPa). When the temperature no longer rises, continue to raise the temperature to 120°C, and keep the temperature for 5h. After the reaction was finished, the temperature was lowered, the layers were separated, and the lower oil phase was taken to obtain 190 g of N-methyl-o-nitroaniline.
[0038] Add 120g of ethanol, 100g of N-methyl o-nitroaniline, 7g of FeCl to the 1L reaction flask 3 , 7g of activated carbon, start stirring, raise the temperature to 70-75°C, keep a slight reflux, slowly add hydrazine hydrate dropwise to it, after the addition is complete, keep the temperature at 80°C for 20h. After the...
Embodiment 3
[0041] Add 200g of o-chloronitrobenzene and 197g of monomethylamine aqueous solution into a 1L autoclave, seal it, pressurize it with nitrogen to 0.5MPa, start to raise the temperature slowly, when T=90°C, stop heating, the reaction enters a spontaneous state, exotherm , the temperature continues to rise (the highest temperature can reach 110°C, and the maximum pressure is 1.2MPa). When the temperature no longer rises, continue to raise the temperature to 120°C, and keep the temperature for 5h. After the reaction was finished, the temperature was lowered, the layers were separated, and the lower oil phase was taken to obtain 190 g of N-methyl-o-nitroaniline.
[0042] Add 120g of ethanol, 100g of N-methyl-o-nitroaniline, and 10g of loaded lacquer nickel finished product in sequence to a 1L reaction bottle, start stirring, raise the temperature to 70-75°C, keep a slight reflux, and slowly add hydrazine hydrate dropwise, After the dropwise addition, keep the reaction at 80°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com