Synthesis method of sulfentrazone intermediate
A synthesis method and technology of sulfentrazone are applied in the field of synthesis of sulfentrazone intermediates, can solve the problems of hazardous waste of sodium chloride, cannot be used well for downstream production, increase production cost, etc., and achieve good energy saving, The effect of avoiding supply constraints and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
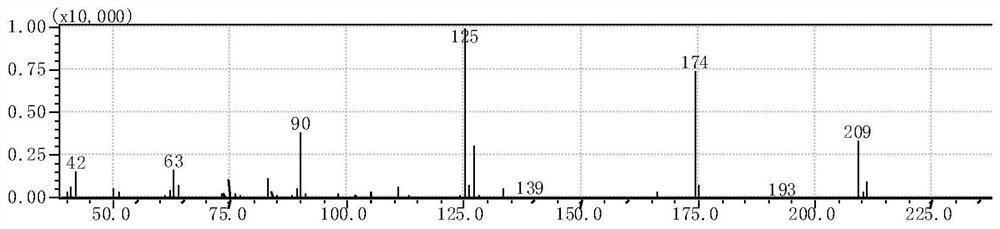
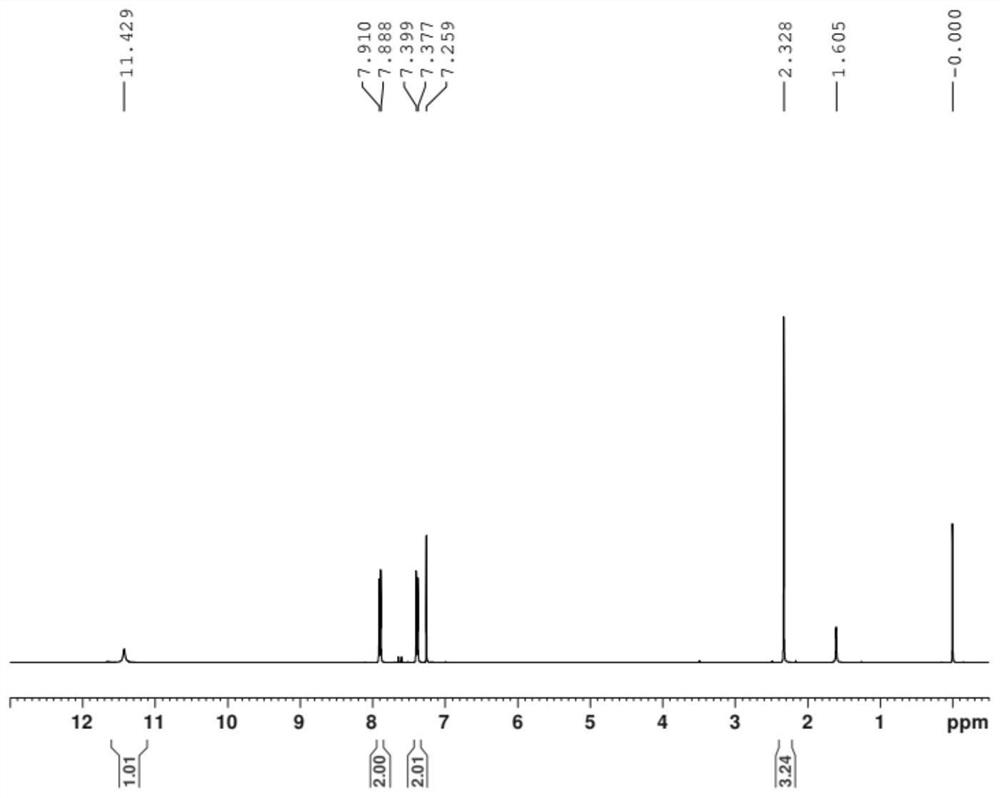
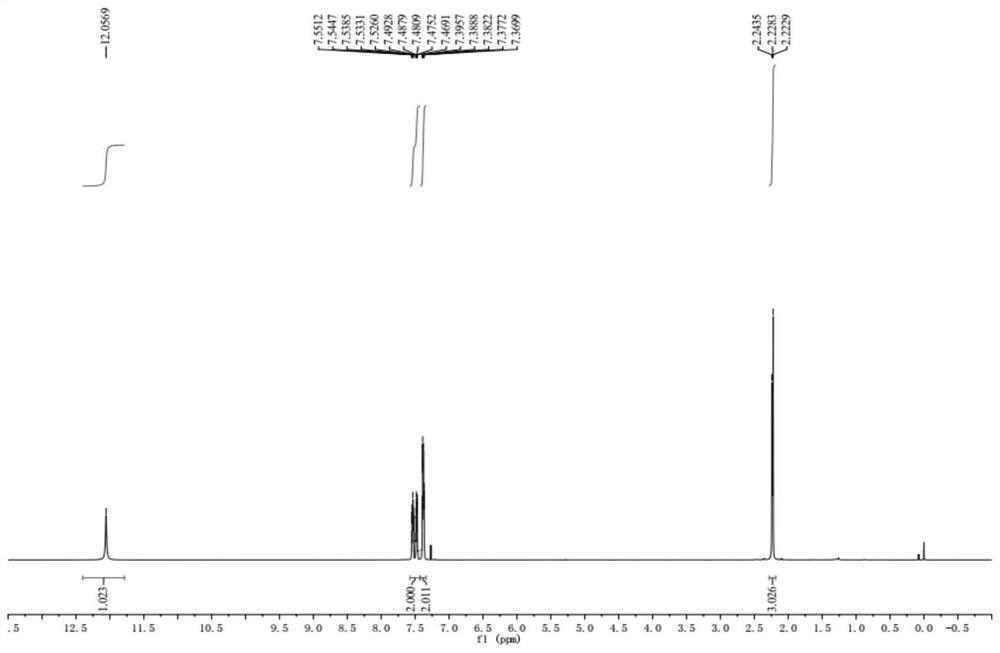
Image
Examples
Embodiment 1
[0079] Synthesis of 1-(2,4-dichlorophenyl)-3-methyl-1H-1,2,4-triazol-5(4H)-one
[0080] The reaction scheme is as follows:
[0081]
[0082] The specific synthesis method is as follows:
[0083] The first step: Chlorobenzene nitration reaction
[0084] The mixture of 300.0g chlorobenzene and 214.0g sulfuric acid nitric acid containing 30% nitric acid was added in the adiabatic nitration reactor, and the adiabatic reaction was started at 25° C. for 1 hour. When the temperature began to drop, the reaction ended. Separation of spent acid, sodium bicarbonate washing to neutrality, decompression distillation to remove chlorobenzene, reclaim chlorobenzene can be recycled, after obtaining o-chloronitrobenzene, p-chloronitrobenzene mixture 150.5g, content 99% (including o-chloronitrobenzene Para-isomer), hereinafter referred to as nitration product I.
[0085] The second step: hydrogenation reduction reaction
[0086] Add 157.5g of nitration product I to 500ml of methanol, add ...
Embodiment 2
[0098] Synthesis of 1-(2,4-dichlorophenyl)-3-methyl-1H-1,2,4-triazol-5(4H)-one
[0099] The reaction scheme is as follows:
[0100]
[0101] The specific synthesis method is as follows:
[0102] The first step: Chlorobenzene nitration reaction
[0103] The mixture of 300.0g chlorinated benzene and 214.0g sulfuric acid nitric acid containing 30% nitric acid was added in the adiabatic nitration reactor, and the adiabatic reaction was started at 25° C. for 1 hour. When the temperature began to drop, the reaction ended. Separation of spent acid, sodium bicarbonate washing to neutrality, decompression distillation to remove chlorobenzene, reclaim chlorobenzene can be recycled, after obtaining o-chloronitrobenzene, p-chloronitrobenzene mixture 150.5g, content 99% (including o-chloronitrobenzene Para-isomer), hereinafter referred to as nitration product I;
[0104] The second step: hydrogenation reduction reaction
[0105] Add 157.5g of nitrated product I to 500ml of methanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com