Synthesis technique of benzotriazole
A technology of benzotriazole and synthesis process, applied in the field of synthesis technology of benzotriazole, can solve the problems of separating products, recovering solvent, the danger of explosion of hydrazine hydrate, large amount of hydrazine hydrate, etc., and achieves convenient recovery. The effect of using, reducing the amount and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
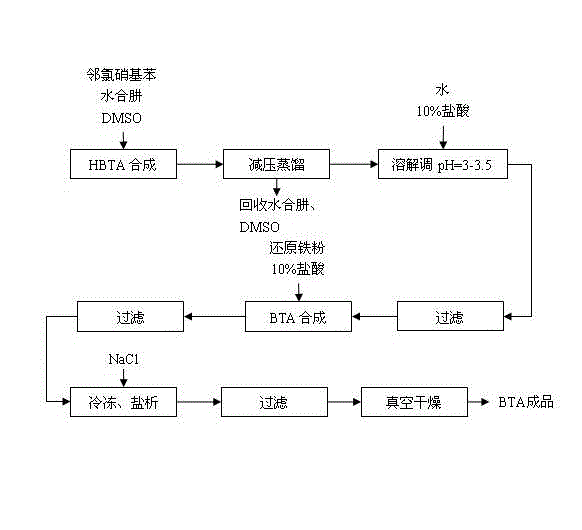
[0020] Embodiment 1, with reference to figure 1 , a kind of synthesis technique of benzotriazole, its concrete steps are as follows:
[0021] (1) In the reaction kettle, add a certain amount of o-nitrochlorobenzene, dimethyl sulfoxide solvent with 0.5 times the mass of o-chloronitrobenzene, and 80% hydrazine hydrate with a molar ratio of two times the o-chloronitrobenzene; heat up to 115 Reflux at ℃ for 3 hours; add 40% NaOH solution in an equimolar amount to o-chloronitrobenzene to fully release the remaining hydrazine hydrate;
[0022] (2) Under reduced pressure, control the vacuum degree to 10kPa, raise the temperature of the oil bath, keep the oil temperature at 75°C for distillation, discard the initial 10% fraction, then collect and recover hydrazine hydrate; and raise the temperature to 135°C to recover dimethyl methoxide Sulfone;
[0023] (3) Add a certain amount of water to the residual liquid to completely dissolve the residue; adjust the pH to 3 with 10% hydrochlo...
Embodiment 2
[0025] Embodiment 2, with reference to figure 1 , a kind of synthesis technique of benzotriazole, its concrete steps are as follows:
[0026] (1) In the reaction kettle, add a certain amount of o-nitrochlorobenzene, dimethyl sulfoxide solvent with 2 times the mass of o-chloronitrobenzene, and 80% hydrazine hydrate with a molar ratio of 5 times the o-chloronitrobenzene; heat up to 115 Reflux at ℃ for 5 hours; add 40% NaOH solution in an equimolar amount to o-chloronitrobenzene to fully release the remaining hydrazine hydrate;
[0027] (2) Under reduced pressure, control the vacuum degree to 20kPa, raise the temperature of the oil bath, keep the oil temperature at 80°C for distillation, discard the initial 10% fraction, then collect and recover hydrazine hydrate; and raise the temperature to 140°C to recover dimethyl methoxide Sulfone;
[0028] (3) Add a certain amount of water to the residual liquid to completely dissolve the residue; adjust the pH to 3.5 with 10% hydrochlori...
Embodiment 3
[0030] Embodiment 3, with reference to figure 1 , a kind of synthesis technique of benzotriazole, its concrete steps are as follows:
[0031] (1) In the reaction kettle, add a certain amount of o-nitrochlorobenzene, dimethyl sulfoxide solvent with 1 times the mass of o-chloronitrobenzene, and 80% hydrazine hydrate with a molar ratio of 3.5 times o-chloronitrobenzene; heat up to 115 Reflux at ℃ for 4 hours; add 40% NaOH solution in an equimolar amount to o-chloronitrobenzene to fully release the remaining hydrazine hydrate;
[0032] (2) Under reduced pressure, control the vacuum degree to 15kPa, raise the temperature of the oil bath, keep the oil temperature at 78°C for distillation, discard the initial 10% fraction, then collect and recover hydrazine hydrate; and raise the temperature to 137°C to recover dimethyl methoxide Sulfone;
[0033] (3) Add a certain amount of water to the residual liquid to completely dissolve the residue; adjust the pH to 3.2 with 10% hydrochloric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com