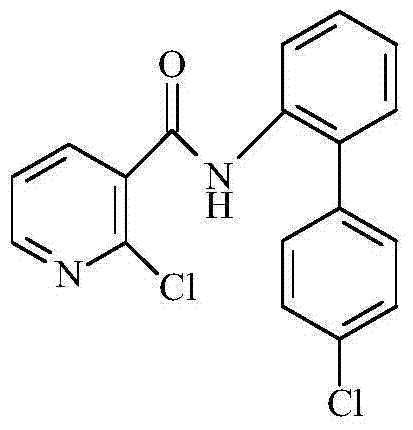
Synthetic method of 2-chloro-N-(4'-chlorodiphenyl-2-yl) nicotinamide
A synthetic method, the technology of chlorinated biphenyls, applied in the direction of organic chemistry, etc., can solve the problems of expensive raw materials, etc., and achieve the effects of mild reaction conditions, cost reduction, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
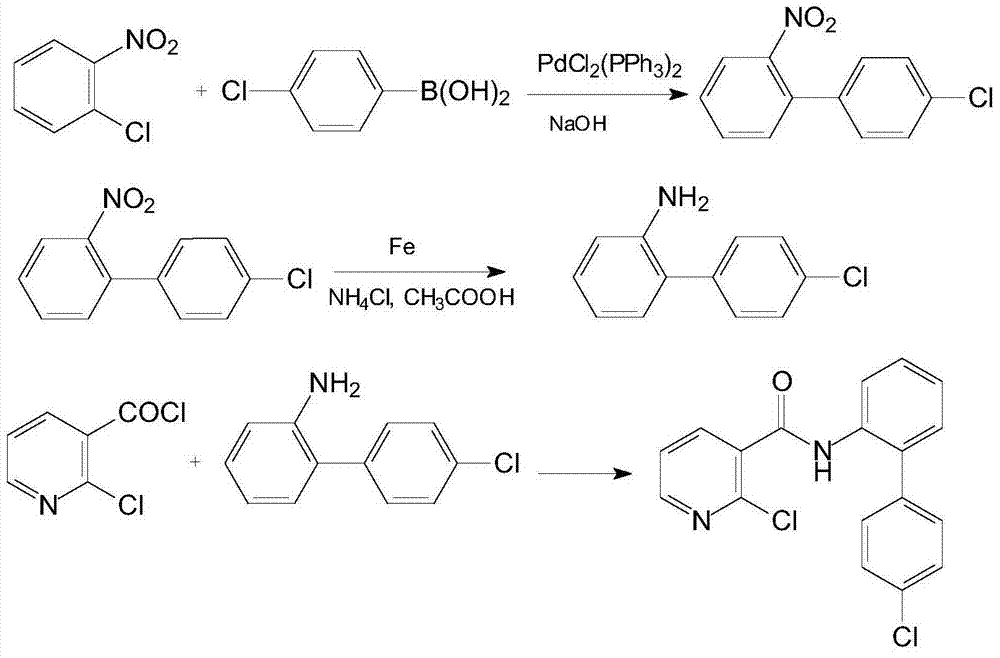
[0027] (1) Add 4.7g (0.03mol) of o-chloronitrobenzene, 3.6g (0.09mol) of sodium hydroxide and 30mL of n-hexane into a 100mL three-necked flask under nitrogen protection, add 0.021g (0.00003mol) of bis( Triphenylphosphine)palladium dichloride, heated up to 50°C, and added dropwise a 30mL n-hexane solution containing 7.0g (0.045mol) of 4-chlorophenylboronic acid. After cooling to room temperature, filter, wash with water until neutral, concentrate, and recrystallize from petroleum ether to obtain 6.3 g of 4'-chloro-2-nitrobiphenyl with a yield of 89.9% and a melting point of 62°C. 1 H NMR (CDCl 3 ), δ: 7.26 (2H, dm, ArH), 7.41 (3H, dm, ArH), 7.51 (1H, dm, ArH), 7.63 (1H, dm, ArH), 7.88 (1H, dd, ArH). MS (m / z): 233 (M + ), 198 (M + -Cl).
[0028] (2) Add 7.0 (0.03mol) g4'-chloro-2-nitrobiphenyl, 8.4g (0.15mol) reduced iron powder, 2.6g (0.045mol) ammonium chloride, 50mL methanol and 10mL water into a 100mL three-necked flask 3.6 g (0.06 mol) of glacial acetic acid was added ...
Embodiment 2
[0032] (1) Under the protection of nitrogen, add 4.7g (0.03mol) o-chloronitrobenzene, 6.7g (0.06mol) potassium tert-butoxide and 30mL chloroform into a 100mL three-necked flask, stir for 10min and then add 0.63g (0.0009mol) bis( Triphenylphosphine)palladium dichloride, heated up to 50°C, and added dropwise a 30mL chloroform solution containing 4.7g (0.03mol) of 4-chlorophenylboronic acid. After cooling to room temperature, filter, wash with water until neutral, concentrate, and recrystallize from petroleum ether to obtain 5.8 g of 4'-chloro-2-nitrobiphenyl with a yield of 82.8%.
[0033](2) Add 7.0 (0.03mol) g4'-chloro-2-nitrobiphenyl, 3.4g (0.06mol) reduced iron powder, 1.8g (0.03mol) ammonium chloride, 50mL ethanol and 10mL water into a 100mL three-necked flask 3.6 g (0.06 mol) of glacial acetic acid was added dropwise at 40° C., and after the addition, the temperature was raised to reflux for 5 h. After cooling to room temperature, filter, concentrate, dissolve with ethyl ...
Embodiment 3
[0037] (1) Add 4.7g (0.03mol) of o-chloronitrobenzene, 3.6g (0.09mol) of sodium hydroxide and 30mL of toluene into a 100mL three-necked flask under nitrogen protection, and add 0.011g (0.000015mol) of bis(tri Phenylphosphine)palladium dichloride, the temperature was raised to 50°C, and a 30mL toluene solution containing 9.4g (0.06mol) 4-chlorophenylboronic acid was added dropwise. After the dropwise addition, the temperature was raised to reflux and kept for 6h. After cooling to room temperature, filter, wash with water until neutral, concentrate, and recrystallize from petroleum ether to obtain 5.0 g of 4'-chloro-2-nitrobiphenyl with a yield of 71.4%.
[0038] (2) Add 7.0 (0.03mol) g4'-chloro-2-nitrobiphenyl, 16.8g (0.3mol) reduced iron powder, 3.5g (0.06mol) ammonium chloride, 50mL ethanol and 10mL water into a 100mL three-necked flask 2.0 g (0.03 mol) of glacial acetic acid was added dropwise at 40° C., and after the addition, the temperature was raised to reflux for 5 h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com