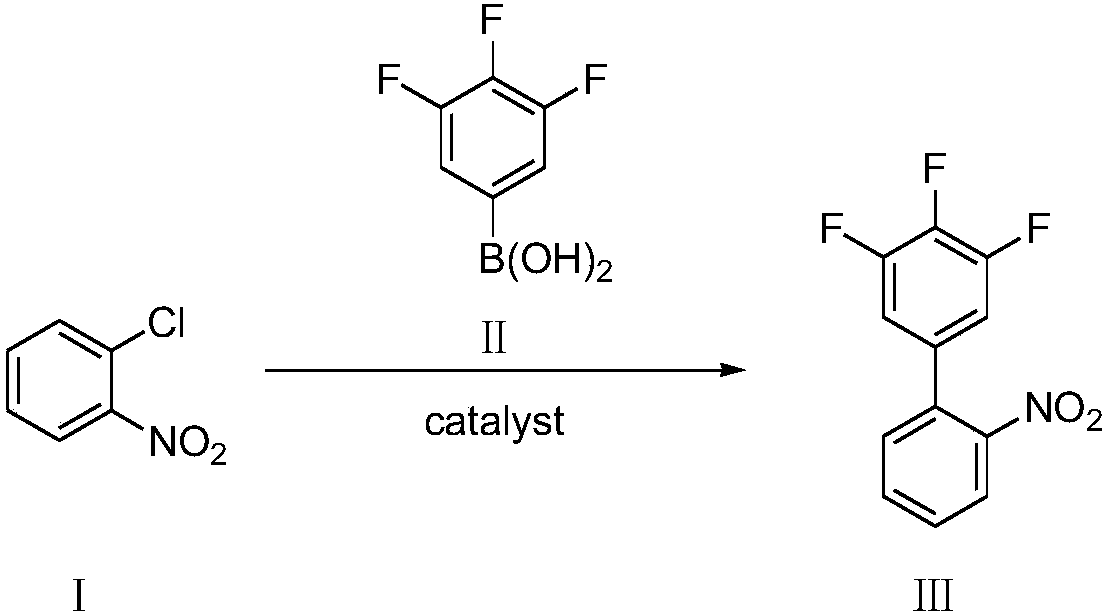
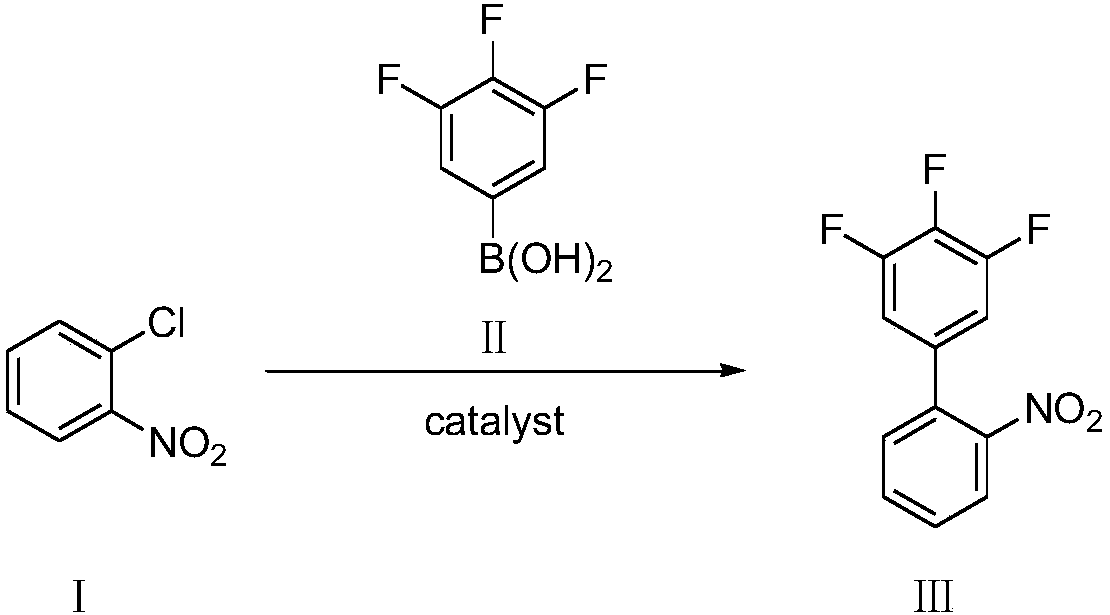
Preparation method of 3,4,5-trifluoro-2'-nitrobiphenyl
A technology of nitrobiphenyl and o-chloronitrobenzene, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as insufficient environmental protection, and achieve improved reaction safety, simple handling, and reduced The effect of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
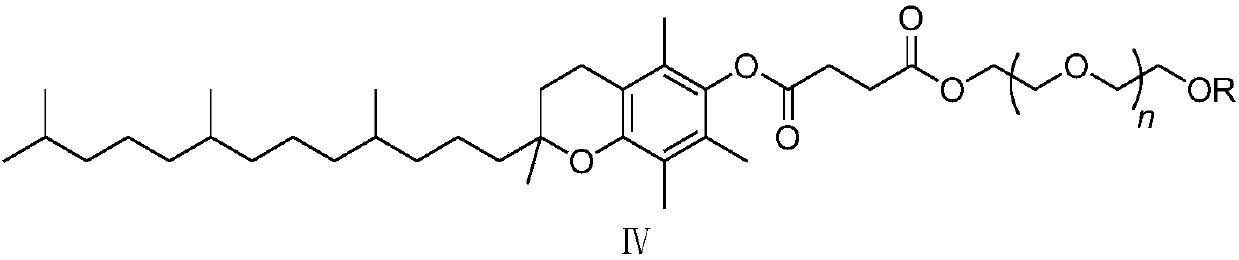
[0048] Add o-chloronitrobenzene (compound Ⅰ) 1.57g, 3,4,5-trifluorophenylboronic acid (compound Ⅱ) 1.94g, palladium chloride 8.8mg (0.5mol%) and SPhos (2-bicyclic Hexylphosphine-2',6'-dimethoxybiphenyl) 41mg (1mol%), add 2% phase transfer catalyst compound IV (wherein R=CH 3 , n=15) of aqueous solution 20mL, replace nitrogen in the system, add 1.66mL (1.2eq) of triethylamine, the system becomes clear, stir and react at 25°C for 8h, there are many solids precipitated in the system, filter, then use 2% HCl Wash with water and dry to obtain 2.15 g of 3,4,5-trifluoro-2'-nitrobiphenyl with a yield of 85% and a purity of 98.5% by HPLC.
Embodiment 2
[0050] Add 1.57 g of o-chloronitrobenzene (compound Ⅰ), 1.94 g of 3,4,5-trifluorophenylboronic acid (compound Ⅱ), 8.8 mg of palladium chloride (0.5 mol%) and XPhos (2-di Cyclohexylphosphonium-2', 4', 6'-triisopropylbiphenyl) 47mg (1mol%), add 2% phase transfer catalyst compound IV (wherein R=CH 3 , n=15) of aqueous solution 20mL, replace nitrogen in the system, add 1.66mL (1.2eq) of triethylamine, the system becomes clear, stir and react at 25°C for 8h, there are many solids precipitated in the system, filter, then use 2% HCl Wash with water and dry to obtain 2.23 g of 3,4,5-trifluoro-2'-nitrobiphenyl with a yield of 88% and a purity of 98.8% by HPLC.
Embodiment 3
[0052]Add 1.57g of o-chloronitrobenzene (compound Ⅰ), 1.94g of 3,4,5-trifluorophenylboronic acid (compound Ⅱ), 11.2mg of palladium acetate (0.5mol%) and SPhos (2-bicyclohexyl Phosphine-2',6'-dimethoxybiphenyl) 41mg (1mol%), add 2% phase transfer catalyst compound IV (wherein R=CH 3 , n=15) of aqueous solution 20mL, replace nitrogen in the system, add 1.66mL (1.2eq) of triethylamine, the system becomes clear, stir and react at 25°C for 8h, there are many solids precipitated in the system, filter, then use 2% HCl Wash with water and dry to obtain 2.14 g of 3,4,5-trifluoro-2'-nitrobiphenyl with a yield of 85% and a purity of 98.1% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com