High dispersed loaded nano-metal Ni catalyst and preparation method thereof
A nano-metal and supported technology is applied in the field of preparation of highly dispersed supported nano-metal Ni catalysts, which can solve the problems of large particle size, collapse of laminates, small specific surface area, etc., and achieve good catalytic performance and improved dispersibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 1 g of carbon nanotubes with a diameter of 40-60 nm and a length of 5-10 μm into 200 ml of acetone and mix them in a four-neck flask, then add 10 ml of acrylic acid, and ultrasonicate at room temperature for 30 minutes under nitrogen protection; then add 0.5 g of azobisiso Butyronitrile was reacted at 55°C for 8h; after cooling to room temperature, suction filtration was repeated, washed with water until neutral, and vacuum-dried at 60°C for 12h to obtain polyacrylic acid surface-functionalized carbon nanotubes for future use.
[0022] A. Weigh 4.3620g of Ni(NO 3 ) 2 ·6H 2 O and 1.8757g of Al(NO 3 ) 3 9H 2 O was dissolved in 50ml deionized water to prepare Ni(NO 3 ) 2 and Al(NO 3 ) 3 mixed salt solution, where Ni(NO 3 ) 2 The concentration is 0.3mol / L, Al(NO 3 ) 3 The concentration is 0.1mol / L; Weigh 2.5600g of NaOH and 2.1198g of NaOH 2 CO 3Dissolve in 100ml deionized water to prepare NaOH and Na 2 CO 3 Mixed alkaline solution, wherein the concentra...
Embodiment 2
[0027] A. with embodiment 1;
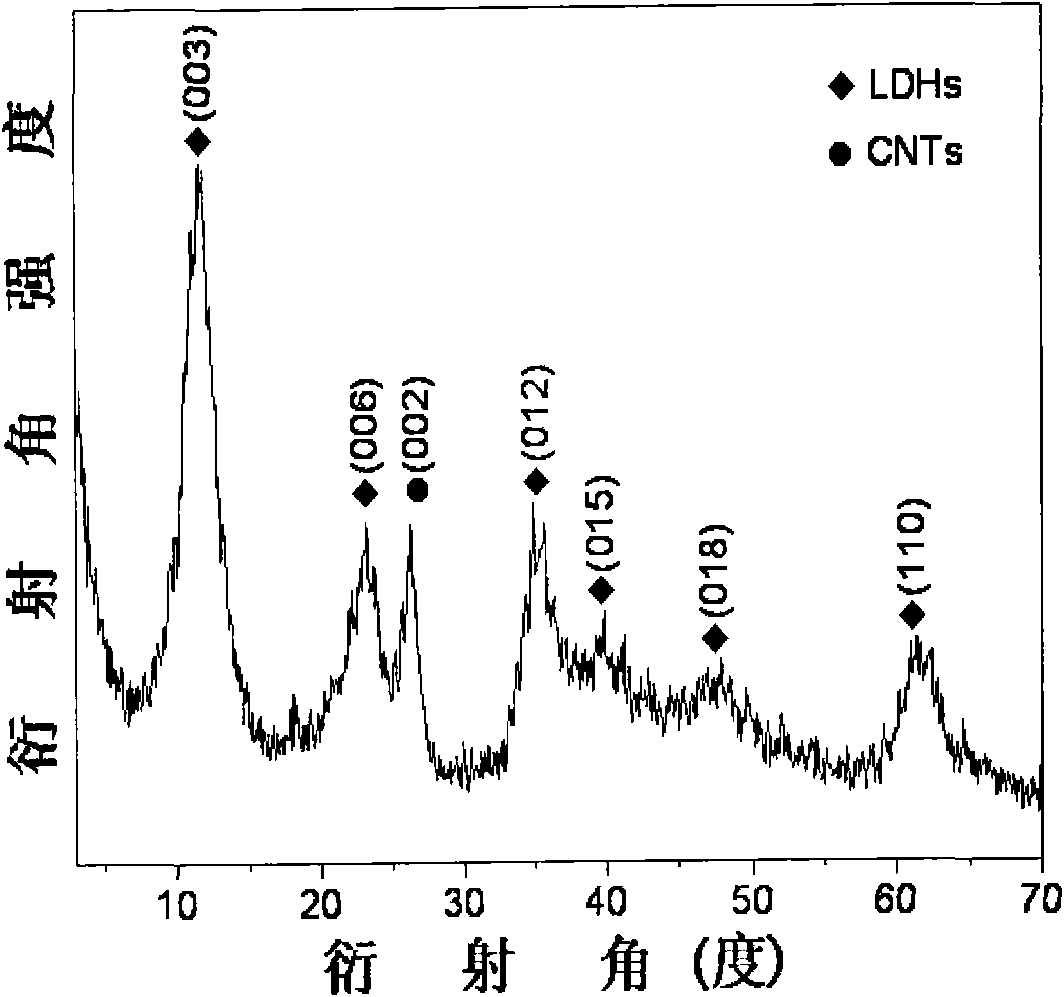
[0028] B. Add the mixed salt solution of 1.0000g of carbon nanotubes and 0.9087g of L-cysteine and 50ml of step A after the method described in Example 1 into the reaction vessel, wherein the bridging agent L-cysteine Amino acid and Ni(NO 3 ) 2 The molar ratio of the substances is 1.0:2, ultrasonically disperse for 30min, then take 100ml of the above mixed alkali solution and add it dropwise into the reaction vessel, adjust the pH of the solution to 10.0, until the mixed alkali solution is added dropwise, then ultrasonically disperse for 30min, and crystallize The temperature is controlled at 60°C, the crystallization time is 6h, and the whole reaction is carried out under N 2 under atmosphere. After the reaction is completed, cool to room temperature, wash the filter cake with deionized water until neutral, and dry it in an oven at 60°C for 12 hours to obtain a NiAl-layered double metal hydroxide / carbon nanotube composite;
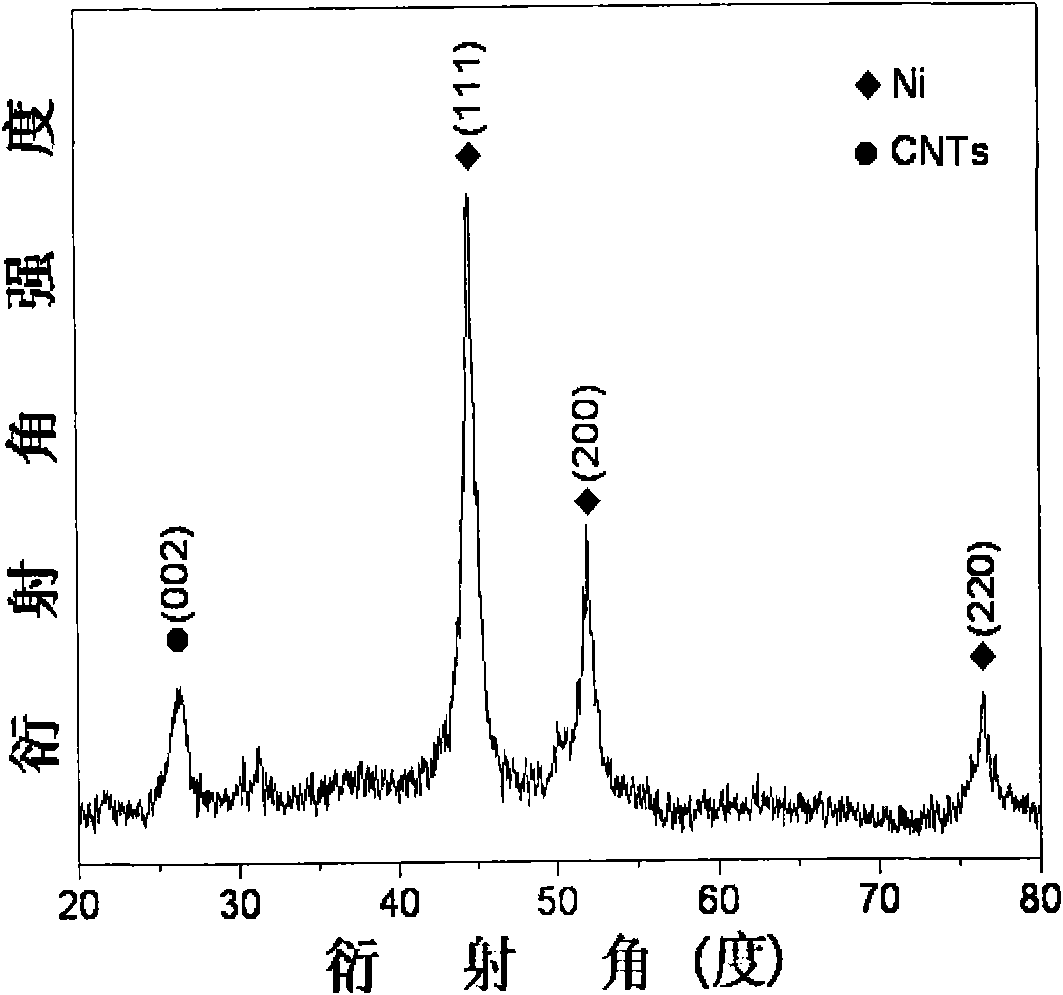
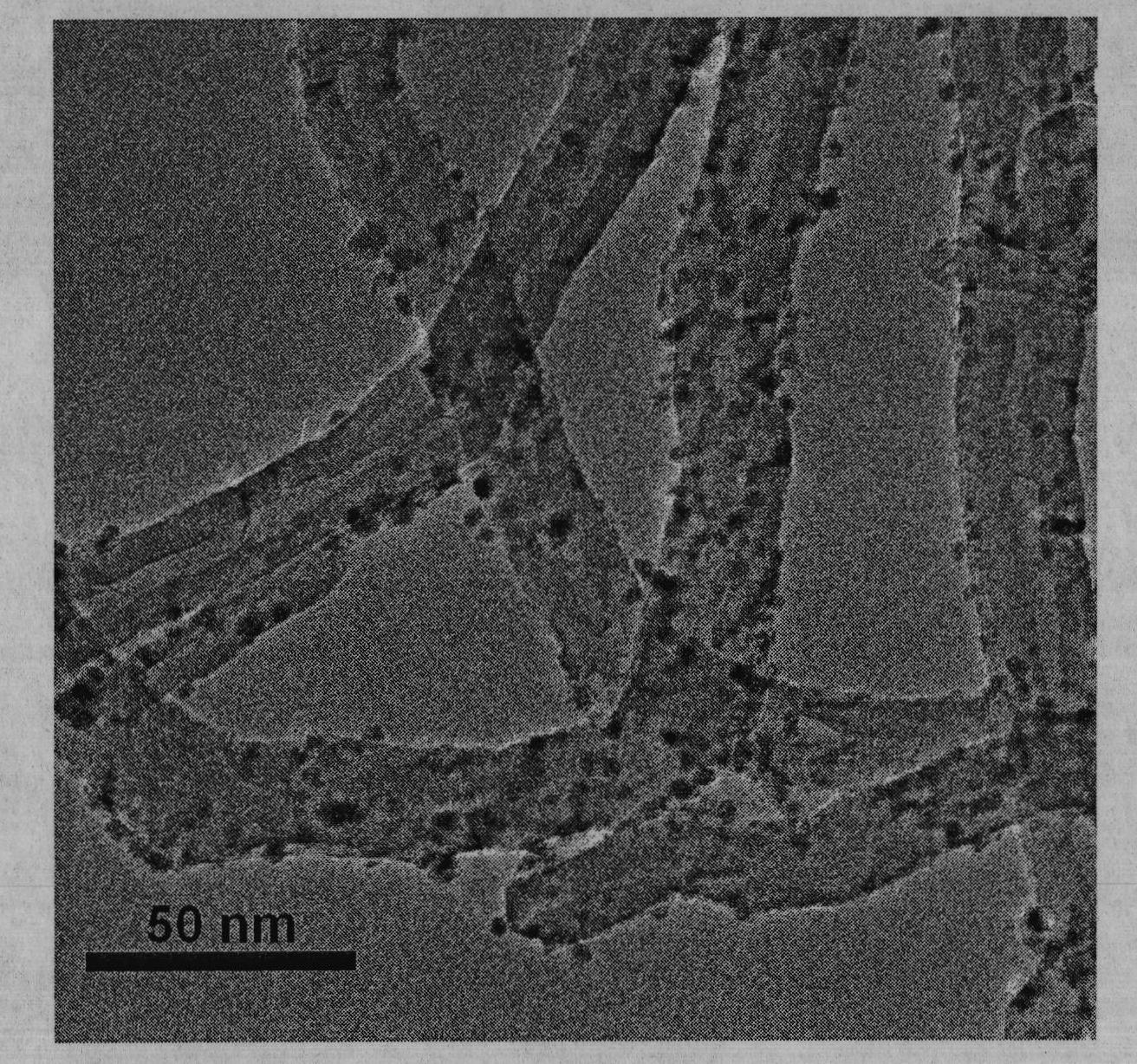
[0029] C. Plac...
Embodiment 3
[0032] A. with embodiment 1;
[0033] B. Add 0.5000g of carbon nanotubes treated according to the method described in Example 1 and 1.8174g of L-cysteine and 50ml of step A mixed salt solution into the reaction vessel, wherein the bridging agent L-cysteine Acid and Ni(NO 3 ) 2 The molar ratio of the substances is 1.0:1, ultrasonically disperse for 40min, then take 100ml of the above mixed alkali solution and add it dropwise into the reaction vessel, adjust the pH of the solution to 11.0, until the mixed alkali solution is added dropwise, then ultrasonically disperse for 40min, and crystallize The temperature is controlled at 80°C, the crystallization time is 12h, and the whole reaction is carried out under N 2 under atmosphere. After the reaction is completed, cool to room temperature, wash the filter cake with deionized water until neutral, and dry it in an oven at 80°C for 18 hours to obtain a NiAl-layered double metal hydroxide / carbon nanotube composite;
[0034] C. P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com