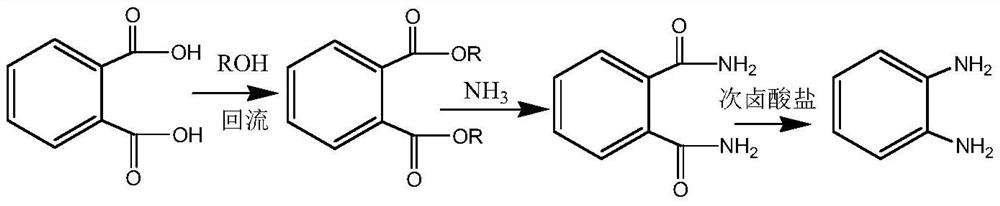
Preparation method of o-phenylenediamine
A technology of o-phenylenediamine and phthalamide, which is applied in the preparation of carboxylic acid amides, organic compounds, and carboxylic esters, and can solve problems such as waste acid generation, casualty economy, and difficult temperature control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 33.2g of phthalic acid to 300ml of methanol, add 1ml of concentrated sulfuric acid, heat up and reflux for 12 hours, and continuously collect fractions below 66°C to remove produced water. When the reflux temperature reaches above 67°C, the esterification is completed, and the remaining methanol, 50ml of ethyl acetate and 20ml of water were added, layered and washed, and after the solvent was distilled, 38.7g of dimethyl phthalate was obtained. The yield is 99.7%.
Embodiment 2
[0032] Add 33.2g of phthalic acid to 300ml of methanol, add 0.5g of p-toluenesulfonic acid, raise the temperature and reflux for 13 hours, and continuously collect fractions below 66°C to remove the produced water. When the reflux temperature reaches above 67°C, the esterification is completed and evaporated Remove remaining methanol, add 50ml of ethyl acetate and 20ml of 0.5% liquid caustic soda, separate layers for washing, and distill to obtain 38.6g of dimethyl phthalate. Yield 99.5%.
Embodiment 3
[0034] Add 33.2g of phthalic acid to 300ml of ethanol, add 3g of solid acid sulfonic acid resin, heat up and reflux for 16 hours, continuously collect fractions below 78°C to remove produced water, when the reflux temperature reaches above 79°C, the esterification is completed, filter , Distilled to obtain 44.2 g of diethyl phthalate. Yield 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com