A kind of preparation method of o-aminothiophenol
A technology of o-aminothiophenol and o-chloronitrobenzene, which is applied in the field of compound preparation, can solve the problems of many reaction by-products, low product purity, and difficulty in obtaining raw materials, and achieve less side reactions, high product yield, and mechanism clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The present embodiment provides a kind of preparation method of o-aminothiophenol, and specific reaction process is as follows figure 1 shown. Specifically include the following steps.
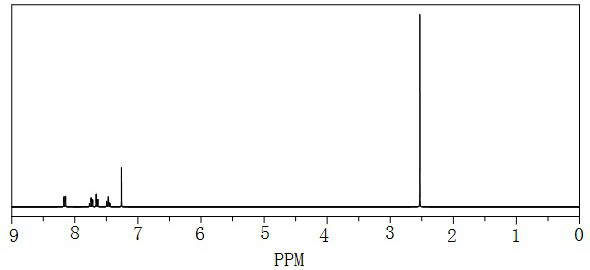
[0046] Add o-chloronitrobenzene: 157.6g (1mol, 1.0eq) to 1000mL, 20% sodium methyl mercaptide aqueous solution: 525g (1.5mol, 1.5eq), tetrabutylammonium bromide: 9.7g (0.03mol, 0.03 eq), start stirring and heat up to 55°C, heat preservation reaction for 6 hours, the control o-chloronitrobenzene in HPLC is 0.6%, the reaction is over, cool down to 20°C, stand for 0.5 hours, and separate layers, the lower layer is o-nitrobenzyl Thioether: 164.0 g, yield: 97.1%, purity 99.3%. The proton nuclear magnetic spectrum of the o-nitroanisole sulfide of the present embodiment- 1 HNMR such as figure 2 as shown, figure 2 As can be seen 1 H NMR (500 MHz, CDCl 3 ), δ=2.52(s, 3H, CH 3 ),7.50(t,1H,A r H), 7.70-7.80 (m, 2H, A r H), 8.21(d, 1H, A r h).
[0047]In a 2000mL autoclave, add o-nitr...
Embodiment 2
[0050] This embodiment provides a preparation method of o-aminothiophenol, which specifically includes the following steps.
[0051] Add o-chloronitrobenzene: 157.6g (1mol, 1.0eq) to 1000mL, 20% sodium methyl mercaptide aqueous solution: 560g (1.6mol, 1.6eq), chain polyethylene glycol: 5.0g, start stirring and heat up to 68 ° C, heat preservation reaction for 5 hours, the control of o-chloronitrobenzene in HPLC is 0.4%, the reaction is completed, the temperature is lowered to 20 ° C, standing for 0.5 hours, and the layers are separated. The lower layer is o-nitroanisole sulfide: 162.5g, collected Yield: 96.2%, purity 99.2%.
[0052] In a 2000mL autoclave, add o-nitroanisole sulfide: 162.5g (0.96mol, 1.0eq), ethanol: 800g, Raney nickel: 1g, pass nitrogen replacement three times, then pass hydrogen replacement three times, and increase the pressure to 1.7MPa, heat up to 65°C for hydrogenation reaction, start the central control when the pressure no longer drops significantly, i...
Embodiment 3
[0055] This embodiment provides a preparation method of o-aminothiophenol, which specifically includes the following steps.
[0056] Add o-chloronitrobenzene: 157.6g (1mol, 1.0eq), 20% sodium methyl mercaptide aqueous solution: 700g (2.0mol, 2.0eq), cyclodextrin: 8.0g to 1000mL, start stirring and heat up to 70°C, Insulation reaction for 5 hours, the control of o-chloronitrobenzene in HPLC was 0.3%, the reaction was completed, the temperature was lowered to 20°C, and stood for 0.5 hours, and the layers were separated. The lower layer was o-nitroanisole sulfide: 163.3g, yield: 96.5 %, 99.5% purity.
[0057] Add o-nitroanisole sulfide: 163.3g (0.965mol, 1.0eq), methanol: 700g, platinum carbon: 1g into a 2000mL autoclave, replace with nitrogen three times, then replace with hydrogen three times, and raise the pressure to 1.5 MPa, heat up to 80°C for hydrogenation reaction, and start central control when the pressure no longer drops significantly. It takes about 5 hours to keep w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com