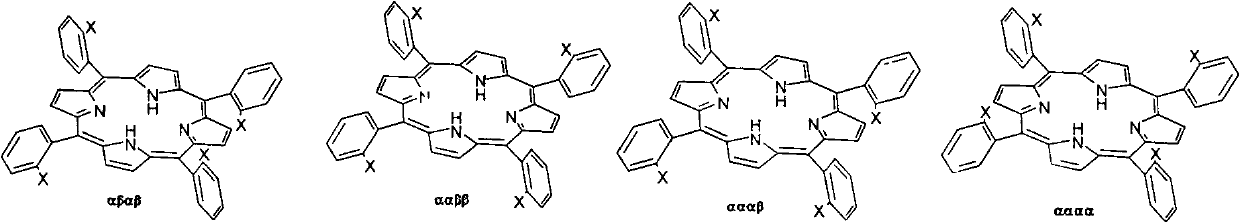
Preparation method for tetra-(o-pivaloyl amino phenyl) zinc protoporphyrin isomer compound
A technology of pivalamidophenyl and nitrophenylporphyrin, which is applied in the field of preparation of four isomer compounds, can solve the problem of easy isomerization, rare and difficult to obtain four pure isomers Compounds and other problems, to achieve the effect of high yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) In a 100ml three-neck flask, add 80ml of concentrated hydrochloric acid, 1.613g of NO 2 PP, 6.721g SnCl 2 2H 2 O, install a reflux condenser, a thermometer, magnetically stir, rapidly raise the temperature to 70°C, react for 30 minutes, stop the reaction, CHCl 3 Extraction and liquid separation, organic phase rotary evaporation to obtain NH 2 The crude PP solid product was separated by column with petroleum ether and ethyl acetate 5:1 as eluent, and different purple bands were collected, and the solvent was removed by rotary evaporation at low temperature to obtain αβαβ-NH 2 PP, ααββ-NH 2 PP, αααβ-NH 2 PP, αααα-NH 2 PP four compounds, a total of 1.334g, yield 92.0%.
[0021] (2) Add 0.507g αβαβ-NH to a 100ml three-neck flask 2 PP, 30mlCH 2 Cl 2 , install a reflux condenser, a thermometer, magnetically stir, cool in an ice-water bath, control the temperature below 5°C, add a little excess pivaloyl chloride and pyridine dropwise, react for one hour, stop the ...
Embodiment 2
[0024] (1) In a 100ml three-neck flask, add 80ml of concentrated hydrochloric acid, 1.659gNO 2 PP, 6.724g SnCl 2 2H 2 O, install a reflux condenser, a thermometer, magnetically stir, rapidly raise the temperature to 70°C, react for 30 minutes, stop the reaction, CHCl 3 Extraction, organic phase rotary evaporation to obtain NH 2 The crude PP solid product was separated by column with petroleum ether and ethyl acetate 5:1 as eluent, and different purple bands were collected, and the solvent was removed by rotary evaporation at low temperature to obtain αβαβ-NH 2 PP, ααββ-NH 2 PP, αααβ-NH 2 PP, αααα-NH 2 PP four compounds, a total of 1.396g, yield 93.1%.
[0025] (2) Add 0.513g αβαβ-NH to a 100ml three-neck flask 2 PP, 30mlCH 2 Cl 2 , install a reflux condenser, a thermometer, magnetically stir, cool in an ice-water bath, control the temperature below 5°C, add a little excess pivaloyl chloride and pyridine dropwise, react for one hour, stop the reaction, and rotate at a ...
Embodiment 3
[0028] (1) In a 100ml three-neck flask, add 80ml of concentrated hydrochloric acid, 1.623g of NO 2 PP, 6.750g SnCl 2 2H 2 O, install a reflux condenser, a thermometer, magnetically stir, rapidly raise the temperature to 70°C, react for 30 minutes, stop the reaction, CHCl 3 Extraction, organic phase rotary evaporation to obtain NH 2 The crude PP solid product was separated by column with petroleum ether and ethyl acetate 5:1 as eluent, and different purple bands were collected respectively, and the solvent was removed by rotary evaporation at low temperature to obtain αβαβ-NH 2 PP, ααββ-NH 2 PP, αααβ-NH 2 PP, αααα-NH 2 PP four compounds, a total of 1.373g, yield 93.6%.
[0029] (2) Add 0.504g ααββ-NH to a 100ml three-neck flask 2 PP, 30mlCH 2 Cl 2 , install a reflux condenser, a thermometer, magnetically stir, cool in an ice-water bath, control the temperature below 5 degrees, add a little excess pivaloyl chloride and pyridine dropwise, react for one hour, stop the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com