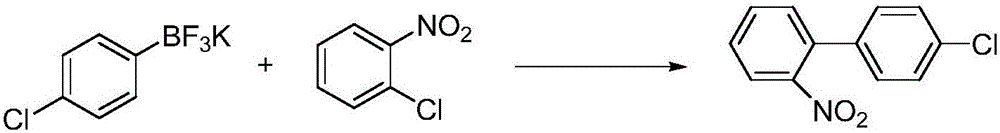Method for preparing 4'-chloro-2-nitrobiphenyl
A technology of nitrobiphenyl and o-chloronitrobenzene, which is applied in the field of preparation of boscalid intermediate 4'-chloro-2-nitrobiphenyl, can solve the problem of low yield, many by-products and difficult separation And other problems, to achieve high yield, simple operation, good product quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
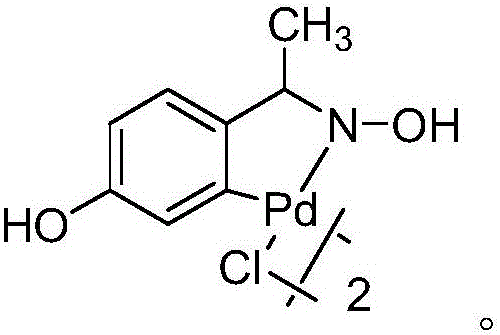
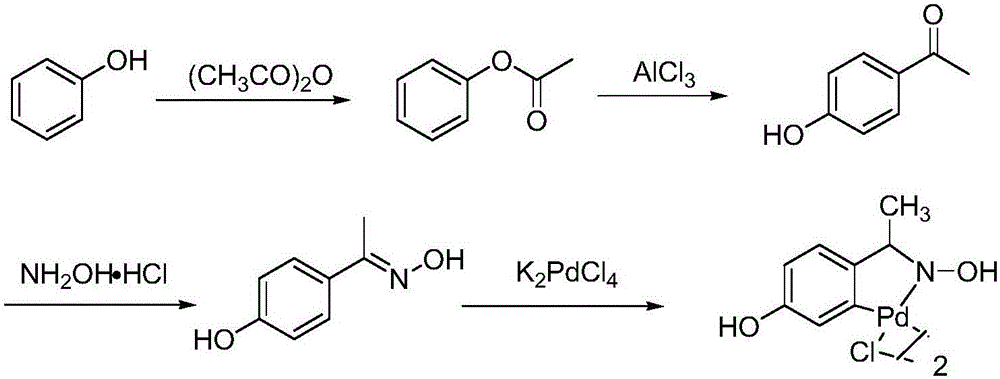
[0027] 15.7g (0.1mol) o-chloronitrobenzene, 28.4g (0.13mol) potassium p-chlorotrifluorophenylborate, 27.6g (0.2mol) potassium carbonate, 16.1g (0.05mol) tetrabutylammonium bromide are added to In a three-necked flask, add 0.035g (0.00006mol) of cyclopalladium compound I, add 1240mL (69mol) of water (molar ratio: o-chloronitrobenzene: potassium p-chlorotrifluorophenyl borate: potassium carbonate: tetrabutyl bromide Ammonium chloride: cyclopalladium compound: water=1:1.3:2:0.5:0.0006:690); the temperature is raised to 100°C and the reaction is refluxed until the o-chloronitrobenzene reaction is complete. The reaction was stopped and cooled to room temperature. Filter, add 500mL dichloromethane to separate the filtrate, extract, and extract the aqueous phase twice with 500mL dichloromethane. Combine the organic phases, dry with anhydrous magnesium sulfate, filter, evaporate the filtrate and add 200mL petroleum ether to the remainder. Stir for 30 minutes, filter, and then recrysta...
Embodiment 2
[0029] 15.7g (0.1mol) o-chloronitrobenzene, 32.7g (0.15mol) potassium p-chlorotrifluorophenylborate, 30.4g (0.22mol) potassium carbonate, 25.7g (0.08mol) tetrabutylammonium bromide were added to In a three-necked flask, add 0.052g (0.00009mol) of cyclopalladium compound I, add 1310mL (73mol) of water (the molar ratio is: o-chloronitrobenzene: potassium p-chlorotrifluorophenyl borate: potassium carbonate: tetrabutyl bromide) Ammonium sulfide: cyclopalladium compound: water=1:1.5:2.2:0.8:0.0009:730); the temperature is raised to 95°C and the reaction is refluxed until the o-chloronitrobenzene reaction is complete. The reaction was stopped and cooled to room temperature. Filter, add 500mL dichloromethane to separate the filtrate, extract, and extract the aqueous phase twice with 500mL dichloromethane. Combine the organic phases, dry with anhydrous magnesium sulfate, filter, evaporate the filtrate and add 200mL petroleum ether to the remainder. Stir for 30 minutes, filter, and the...
Embodiment 3
[0031] 15.7g (0.1mol) o-chloronitrobenzene, 28.4g (0.13mol) potassium p-chlorotrifluorophenylborate, 26.2g (0.19mol) potassium carbonate, 19.3g (0.06mol) tetrabutylammonium bromide are added to In a three-neck flask, add 0.029g (0.00005mol) of cyclopalladium compound I, add 1280mL (71mol) of water (molar ratio: o-chloronitrobenzene: p-chlorotrifluorophenyl borate potassium: potassium carbonate: tetrabutyl bromide Ammonium chloride: cyclopalladium compound: water=1:1.3:1.9:0.6:0.0005:710); the temperature is raised to 100°C and the reaction is refluxed until the o-chloronitrobenzene reaction is complete. The reaction was stopped and cooled to room temperature. Filter, add 500mL dichloromethane to separate the filtrate, extract, and extract the aqueous phase twice with 500mL dichloromethane. Combine the organic phases, dry with anhydrous magnesium sulfate, filter, evaporate the filtrate and add 200mL petroleum ether to the remainder. Stir for 30 minutes, filter, and then recryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com