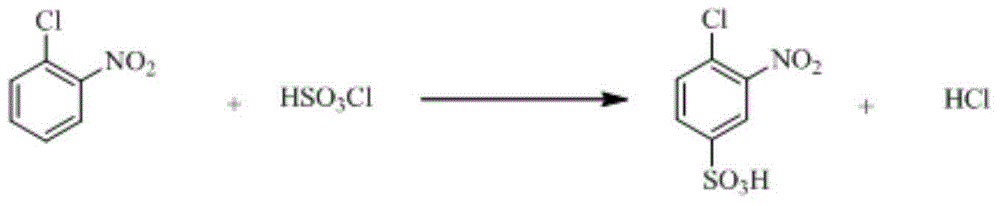
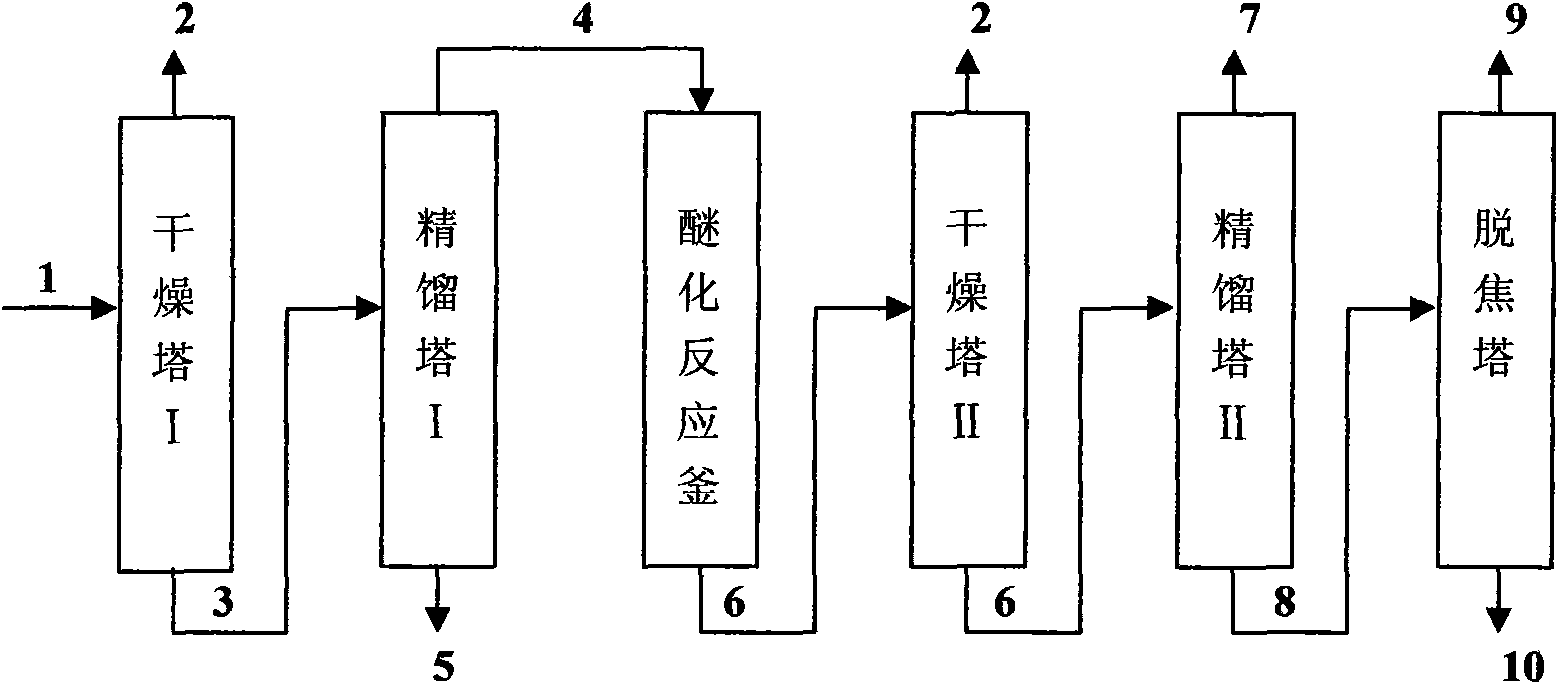
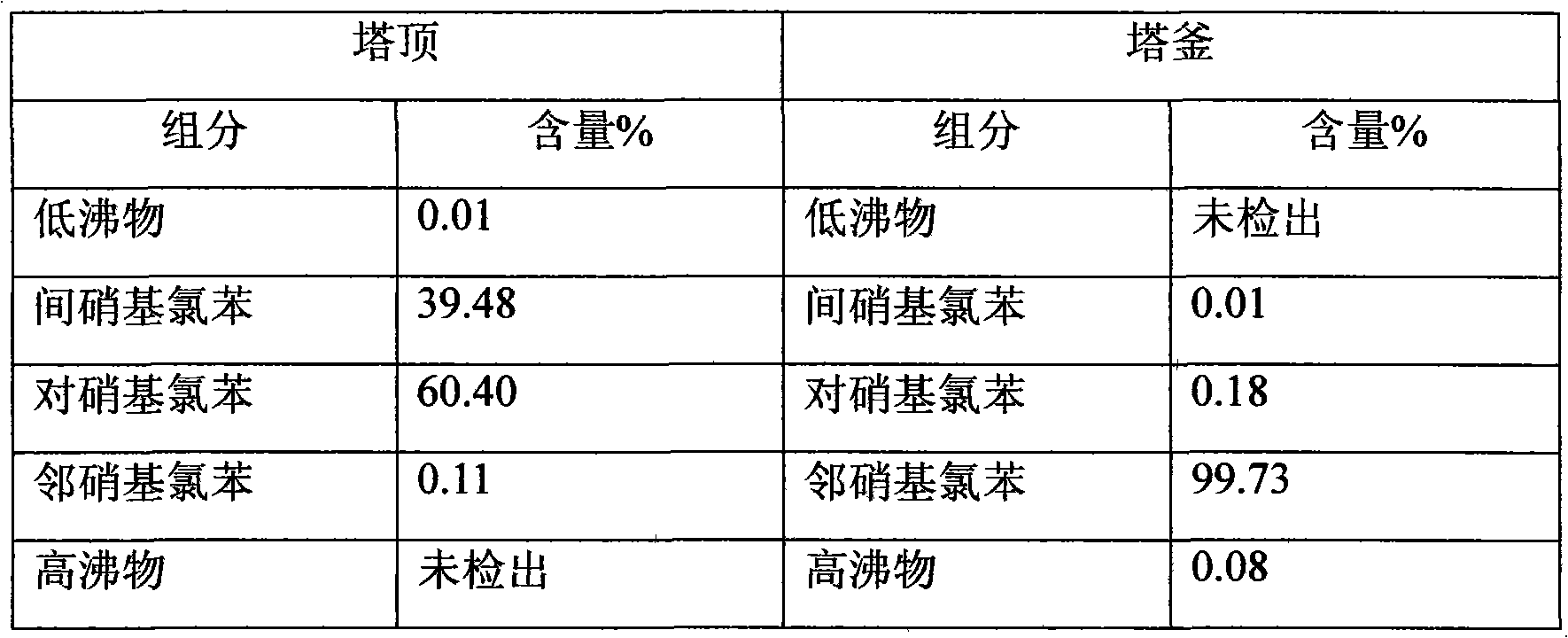
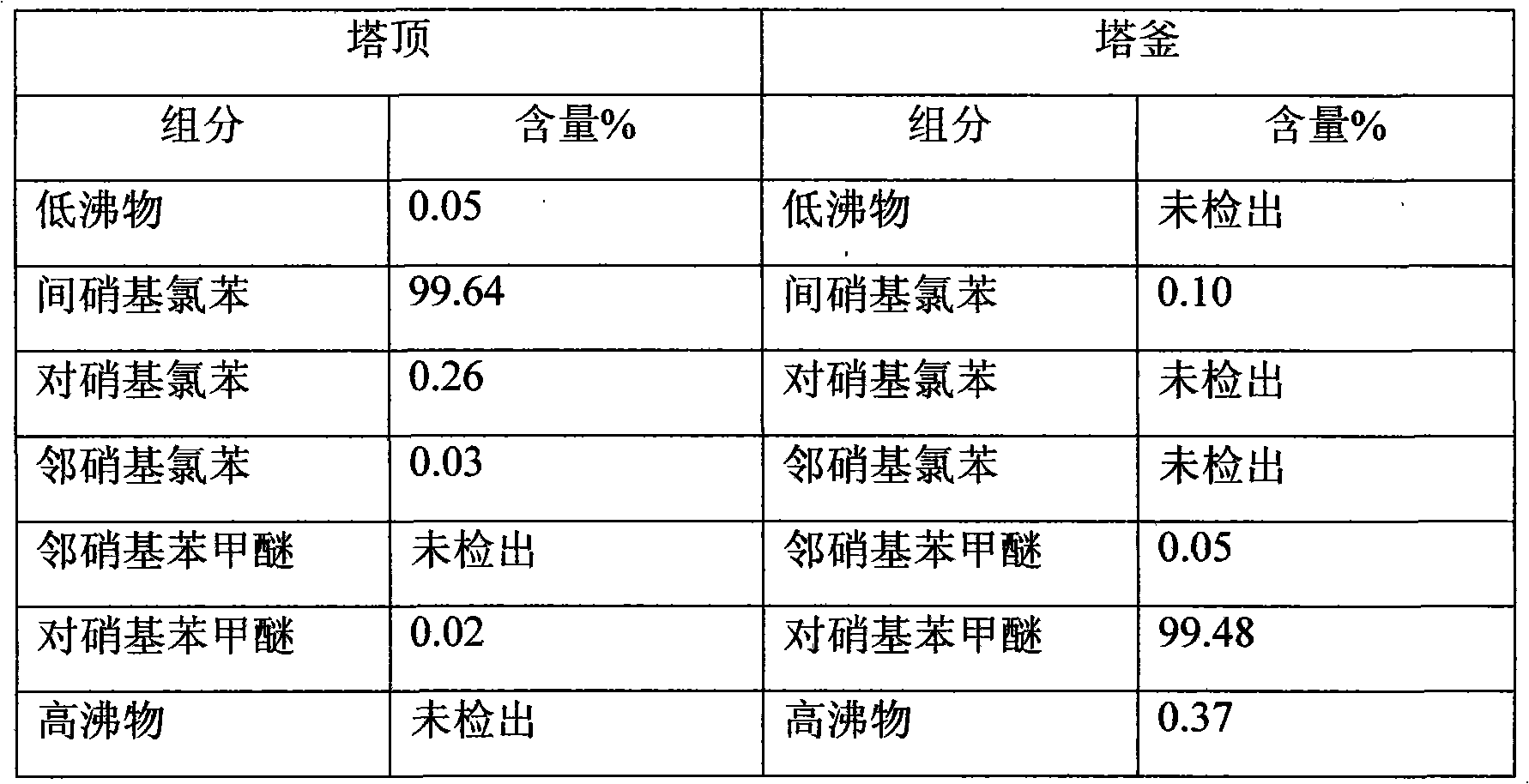
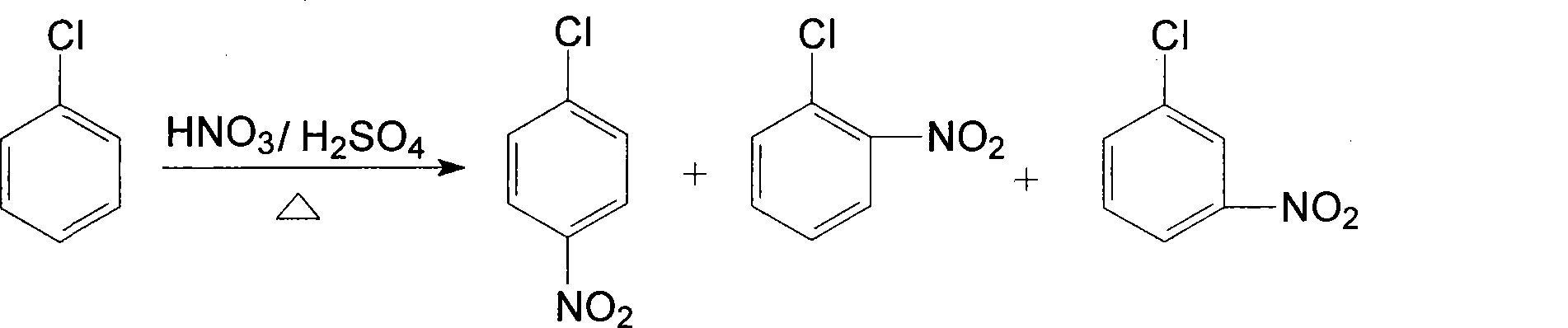
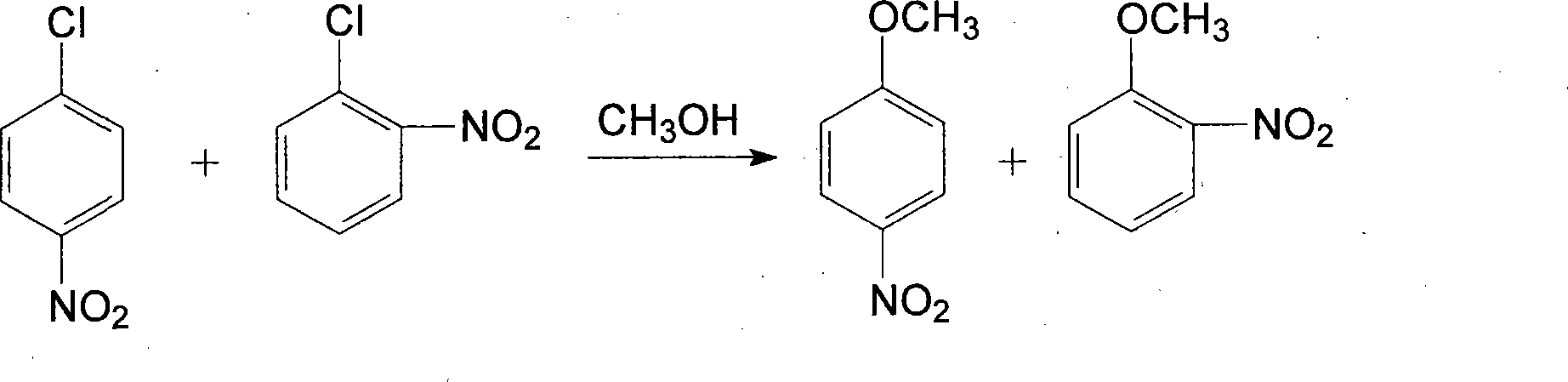
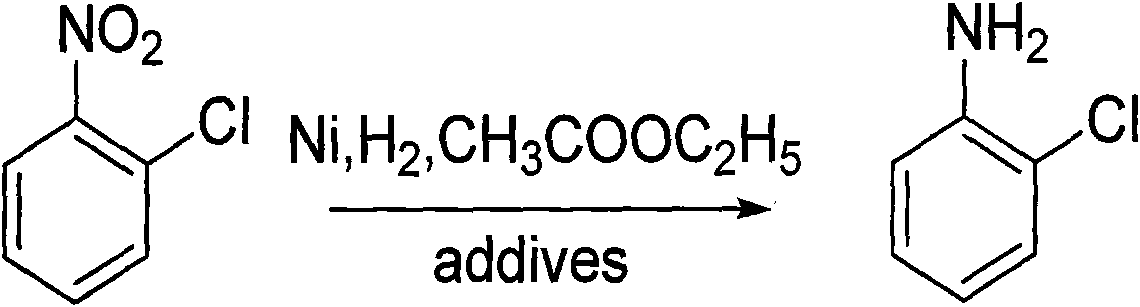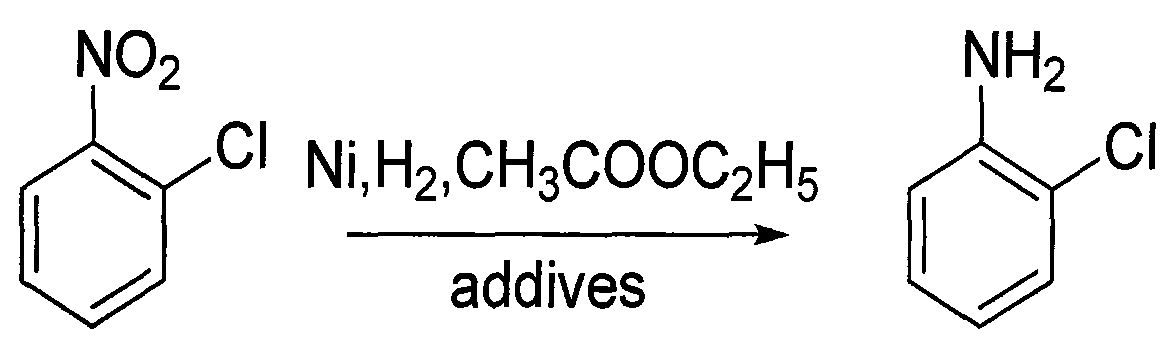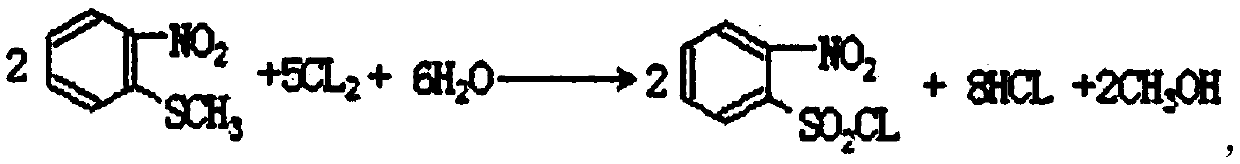Patents
Literature
81 results about "O-nitrochlorobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for producing o-chloroaniline
InactiveCN101333169AHigh yieldLow costOrganic compound preparationAmino compound preparationEthylenediamineO-nitrochlorobenzene
An o-chloroaniline production method takes o-nitrochlorobenzene as raw material and is characterized in that the o-nitrochlorobenzene is dissolved in alcohol solvent in the presence of catalyst and additive and reacted with hydrogen at 10-120 DEG C and under 0.3-4.0 MPa; the reaction process is continuous reaction; after the completion of the reaction, the o-chloroaniline is obtained through treatment, wherein, the catalyst can be selected from one of the following: Ni / Al2O3, Raney Ni, Pt / C and Pd / C; while the additive can be selected from one, or two, or three of the following compounds: cyclohexylamine, ethylenediamine, ethanolamine, diethanolamine, triethanolamine, pyridine, liquid ammonia, ammonium bicarbonate, ammonium carbonate, sodium carbonate, sodium bicarbonate, potassium bicarbonate, potassium carbonate, potassium hydrogen phosphate, potassium dihydrogen phosphate, sodium hydrogen phosphate and sodium dihydrogen phosphate; the dosage of the catalyst takes up 0.05% to 20% of the mass of the o-nitrochlorobenzene; the dosage of the additive takes up 0. 5% to 20% of the mass of the o-nitrochlorobenzene; the alcohol can be methanol or ethanol; the dosage of alcohol takes up 30% to 150% of the dosage of the o-nitrochlorobenzene; the continuous reaction is realized through 1 to 6 tank reactors which are connected in series.
Owner:淮安嘉诚高新化工股份有限公司
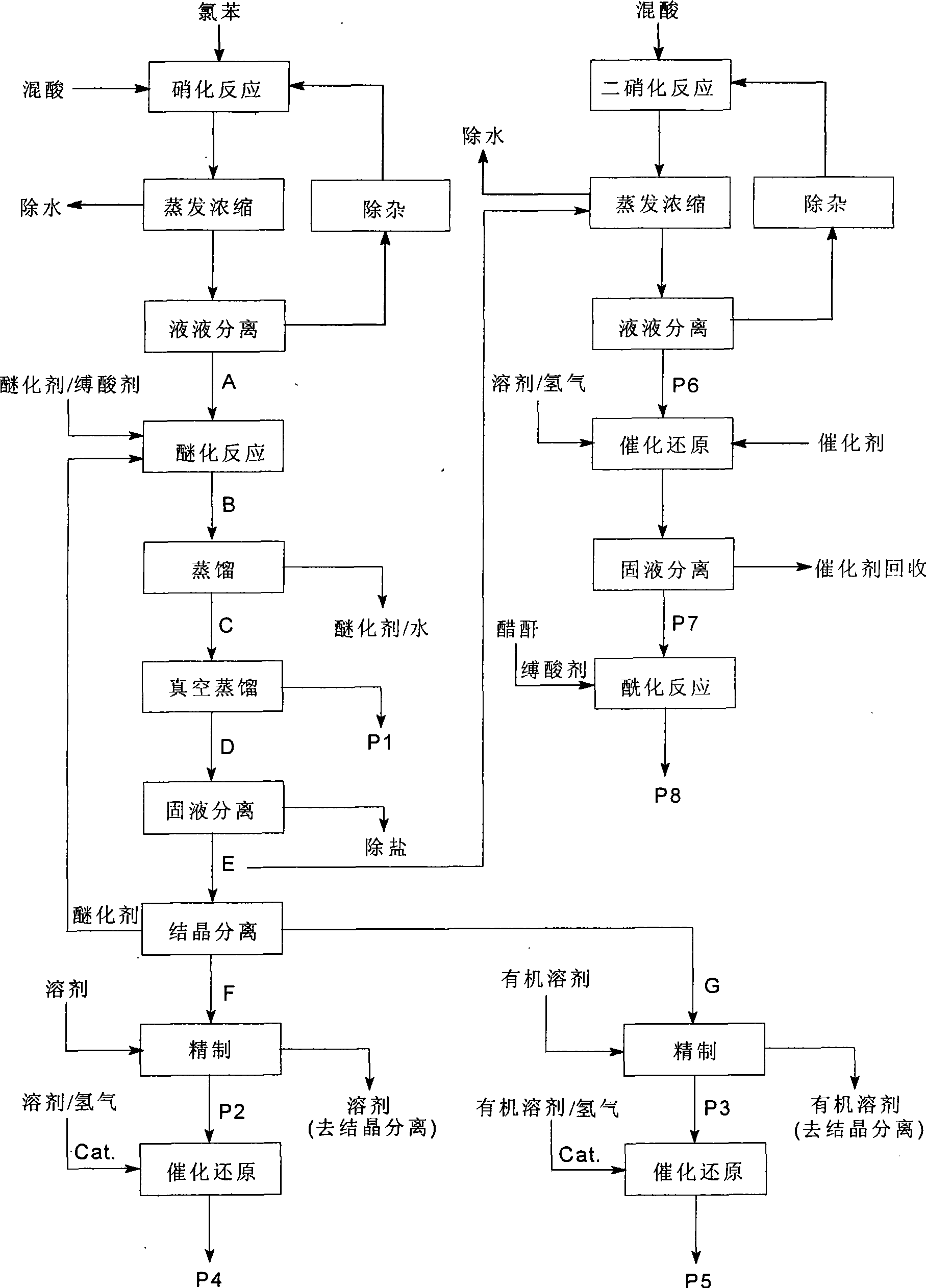
Process of producing nitrobenzether aminobenzether amidobenzether from chlorobenzene
InactiveCN1861562AAvoid conditionsAvoid investment in equipmentOrganic compound preparationCarboxylic acid amides preparationO-nitrochlorobenzeneChloride salt
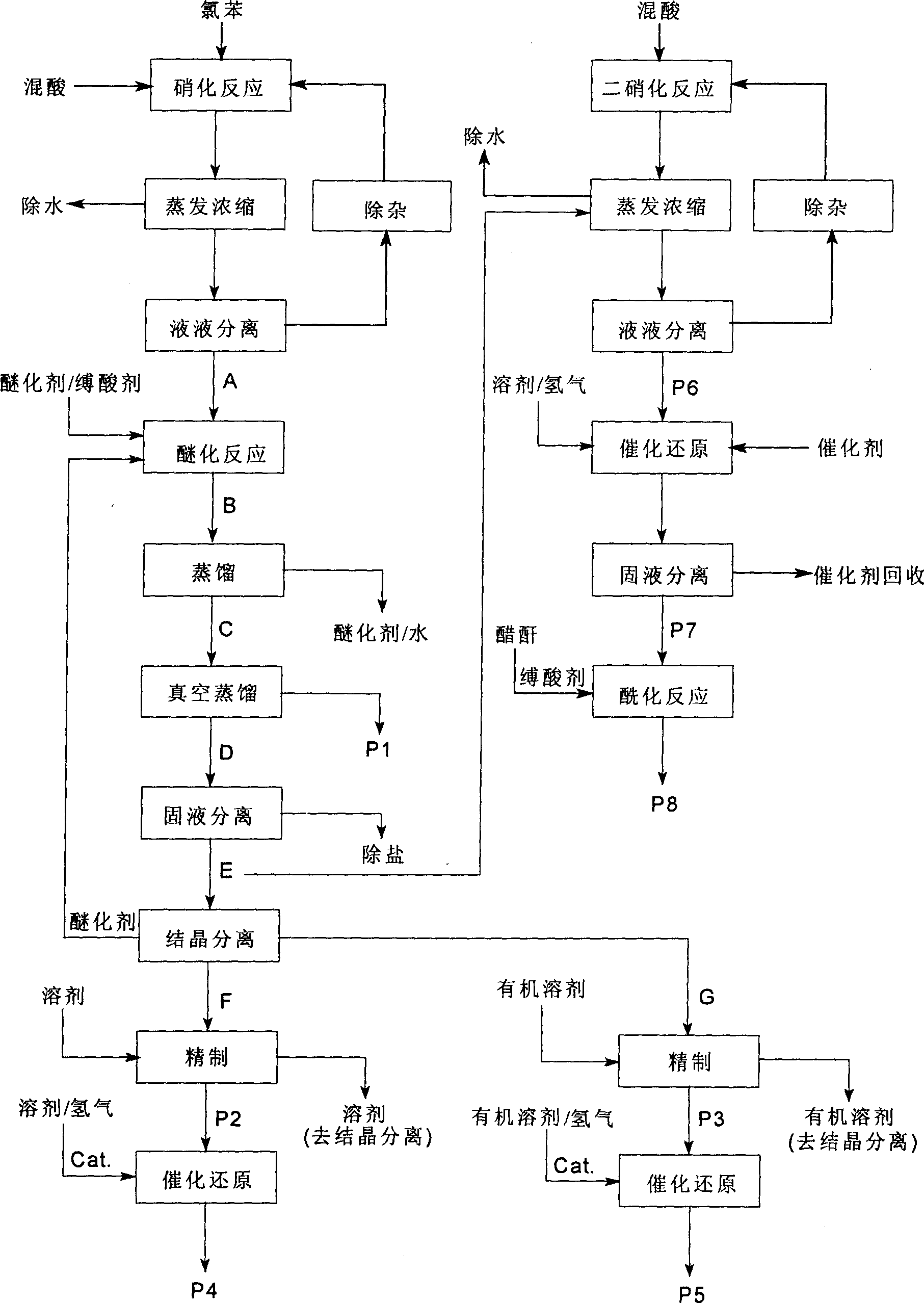
A process for preparing nitrophenylether, aminophenylether and amidophenylether between chlorobenzene and acid mixture removing water, liquid-liquid separation to obtain organic phase consisting of o-, p- and meta-nitro chlorobenzene compounds, etherifying said mixture, recovering etherifying agent, vacuum distilling, separating and refining meta-nitro chlorobenzene, removing chloride salt generated in etherification, crystallizing separation, recrystallizing p- and o-nitro phenylether compounds, catalytic hydrogenating reaction, nitrifying reaction to obtain 2,4-drinitro phenylether, catalytic hydroreducing reaction to obtain 2,4-diamino phenylether, and aceylating reaction to obtain 2-amino-4-acetylamino phenylether.
Owner:CHANGZHOU JIASEN CHEM +1
A kind of production method of anthranil
ActiveCN102276483AReduce consumptionSimple processOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneSodium methoxide
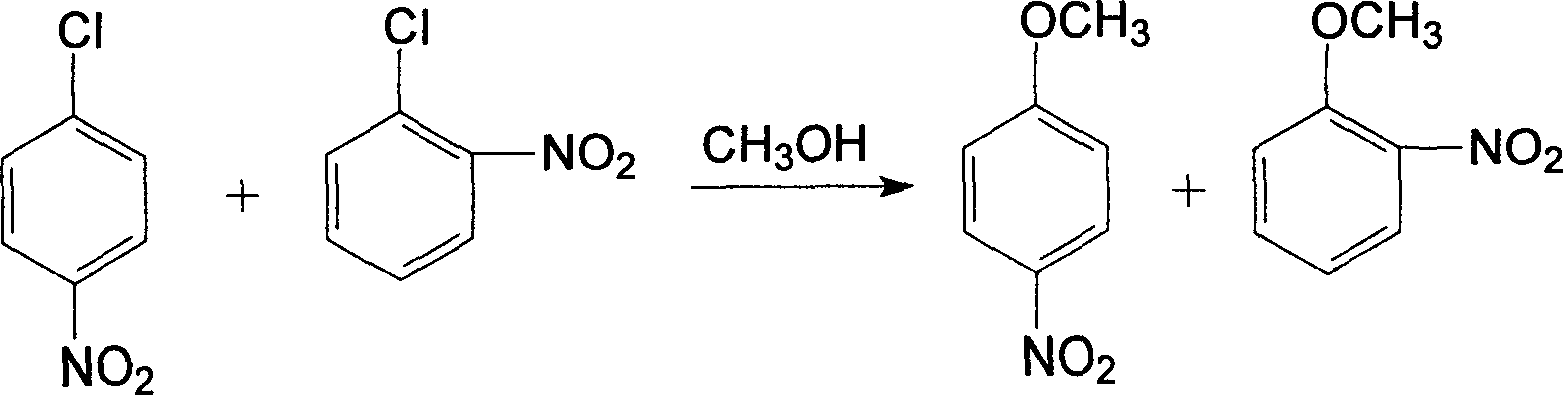
The invention provides a production method of oamino pheylmethyl ether, and the method comprises the following steps of: reacting ortho nitrochlorobenzene serving as a raw material with sodium methoxide for carrying out a methoxylation reaction to obtain orthonitroanisole; secondly, carrying out hydrogenation reduction on the orthonitroanisole by using methanol as a solvent in the presence of a catalyst to prepare the oamino pheylmethyl ether; and finally, carrying out dealcoholization, dehydrogenation and refining on reactants to obtain the oamino pheylmethyl ether as a finished product. The production process comprises the following steps of: preparing sodium methoxide, etherifying the ortho nitrochlorobenzene, distilling the methanol and nitroether for separation, hydrogenating the orthonitroanisole, distilling a hydrogenating solution for separating the oamino pheylmethyl ether and treating wastewater. The production method has the characteristics of simple process, short procedure, continuity in reaction, high production efficiency, good product quality, less energy consumption, concentrated purification of reaction wastewater and no emission, and is suitable for producing the oamino pheylmethyl ether by using the ortho nitrochlorobenzene as the raw material.
Owner:LIAONING SHIXING PHARMA & CHEM
Method for preparing o-chloroaniline by catalytic hydrogenation
InactiveCN101774931AEasy to clean regularlyInhibition of dechlorination reactionOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneFixed bed
The invention discloses a method for preparing o-chloroaniline by catalytic hydrogenation. O-nitrochlorobenzene, ethyl acetate solution, a dechlorination inhibitor and hydrogen are subjected to catalytic hydrogenation synthetic reaction under the effect of a catalyst at the temperature of 25-90 DEG C to obtain the o-chloroaniline. For better technical effects, the o-nitrochlorobenzene, ethyl acetate solution, dechlorination inhibitor are continuously delivered to a fixed bed reactor by a metering pump, inlet amount of hydrogen is controlled by a flow meter, and catalytic hydrogenation synthetic reaction is conducted in the fixed bed reactor. The fixed bed reactor which uses Ni alloy as the catalyst and a neutral carrier as a catalyst carrier is used for controlling the inlet amount of raw materials and hydrogen and the catalyst, thus reducing cost and improving efficiency. In the invention, dechlorination reaction can be effectively inhibited, subsequent processing steps of the principal product are simplified, coupling compounds generated in reaction are little, the selectivity of o-chloroaniline is above 99%, and the dechlorinating amount is below 0.3%. The invention has the advantages of mass industrial production and environment protection.
Owner:JIANGSU KANGHENG CHEM
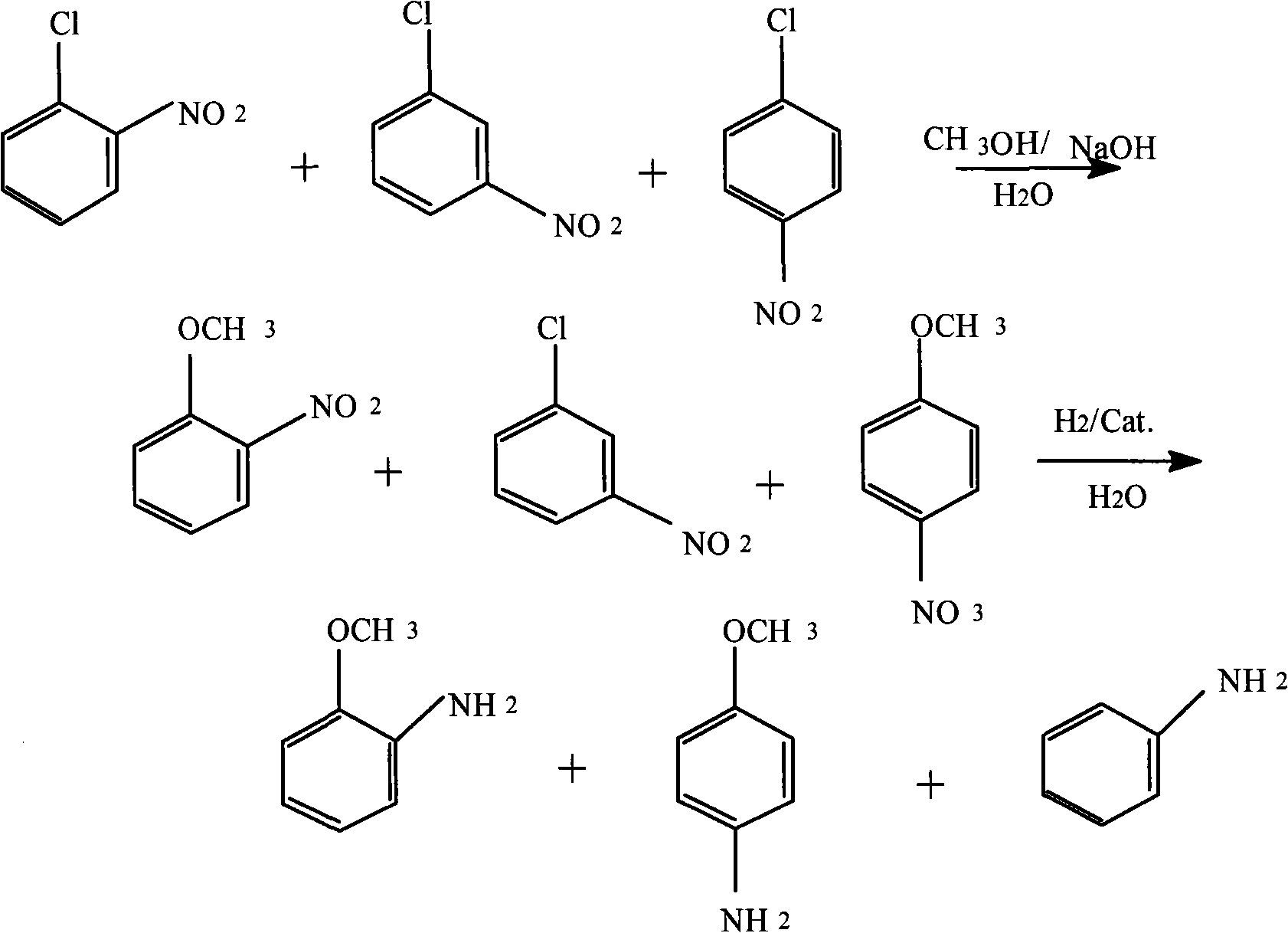
Method for producing anisidine by mixed nitrochlorobenzene reacting in aqueous solvent
InactiveCN101607919ALow ingredient requirementsReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneDistillation
The invention relates to a method for producing anisidine by mixed nitrochlorobenzene (comprising o-nitrochlorobenzene, p-nitrochlorobenzene and m-nitrochlorobenzene) in an aqueous solvent through steps of etherification, hydrogenation, distillation separation, and the like. The method comprises the technical processes: (1) enabling the mixed nitrochlorobenzene and methanol to react, using water as a solvent and sodium hydroxide as a catalyst; (2) separating an aqueous phase; (3) catalyzing and hydrogenating etherified oil, and directly hydrogenating and reducing the etherified oil by using water as the solvent without washing to remove alkaline by water; (4) filtering the catalyst; (5) separating crude products, cooling and precipitating an organic phase, and separating and removing the water phrase; and (6) rectifying and separating an organic phase, and rectifying the organic phase obtained by separating water to obtain pure p-anisidine and pure o-anisidine with the purity over 99 percent. The method for producing anisidine by mixed nitrochlorobenzene reacting in an aqueous solvent has simple technology, low cost and energy consumption, high product purity, environmental protection and low toxicity.
Owner:扬州铭睿达化工科技有限公司 +1
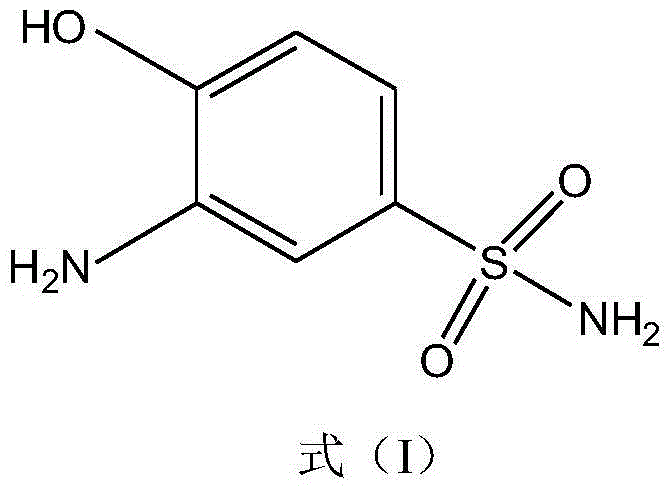
Synthetic method of 2-aminophenol-4-sulfonamide
InactiveCN104592064AReduce dosageReduce wasteOrganic compound preparationSulfonic acid amide preparationO-nitrochlorobenzeneChlorosulfuric acid
The invention relates to a synthetic method of 2-aminophenol-4-sulfonamide. The synthetic method comprises the steps of chlorosulfonation, ammoniation, hydrolysis, acidification and reduction, wherein the chlorosulfonation comprises the following two steps: (a) adding chlorosulfonic acid into p-nitrochlorobenzene which serves as a raw material, and carrying out sulfonation reaction to obtain 4-chloro-3-nitrobenzene sulfonic acid; and (b) adding sulfoxide chloride into 4-chloro-3-nitrobenzene sulfonic acid obtained in the step (a), and carrying out chlorination to obtain 4-chloro-3-nitrobenzenesulfonyl chloride. According to the synthetic method, a chlorosulfonation process is improved, and the chlorosulfonation is carried out in two steps, namely p-nitrochlorobenzene is taken as the raw material, is firstly sulfonated by chlorosulfonic acid and is then chloridized by sulfoxide chloride, so that the consumption of chlorosulfonic acid is reduced, the unnecessary wasting is reduced, the production cost is saved, and the yield of products is increased to reach up to 96.88%.
Owner:QINGDAO DOUBLE PEACH SPECIALTY CHEM GRP
Method for preparing m-nitrochlorobenzene, o-nitrochlorobenzene and p-nitrochlorobenzene by using nitrochlorobenzene meta-position oil
InactiveCN101935281AAvoid pollutionEasy to separateOrganic chemistryOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
The invention relates to a method for preparing m-nitrochlorobenzene, o-nitrochlorobenzene and p-nitrochlorobenzene by using nitrochlorobenzene meta-position oil. The method comprises the following steps of: separating the nitrochlorobenzene meta-position oil containing 20 to 45 percent of m-nitrochlorobenzene and produced in the conventional nitrochlorobenzene production process into an m-nitrochlorobenzene and p-nitrochlorobenzene mixture and an o-nitrochlorobenzene product by a rectification method, further converting the m-nitrochlorobenzene and p-nitrochlorobenzene mixture into an m-nitrochlorobenzene and p-nitroanisole mixture by methoxylation reaction, and performing rectification, separation and purification on the mixture to obtain m-nitrochlorobenzene and p-nitroanisole products. The method has the advantages of low energy consumption, short production period and capability of solving the atmospheric pollution problem of a burning treatment method for the nitrochlorobenzene meta-position oil.
Owner:邵阳市申强化工有限责任公司
Method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene
InactiveCN103073431AAvoid costly separationsSave energyOrganic chemistryOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
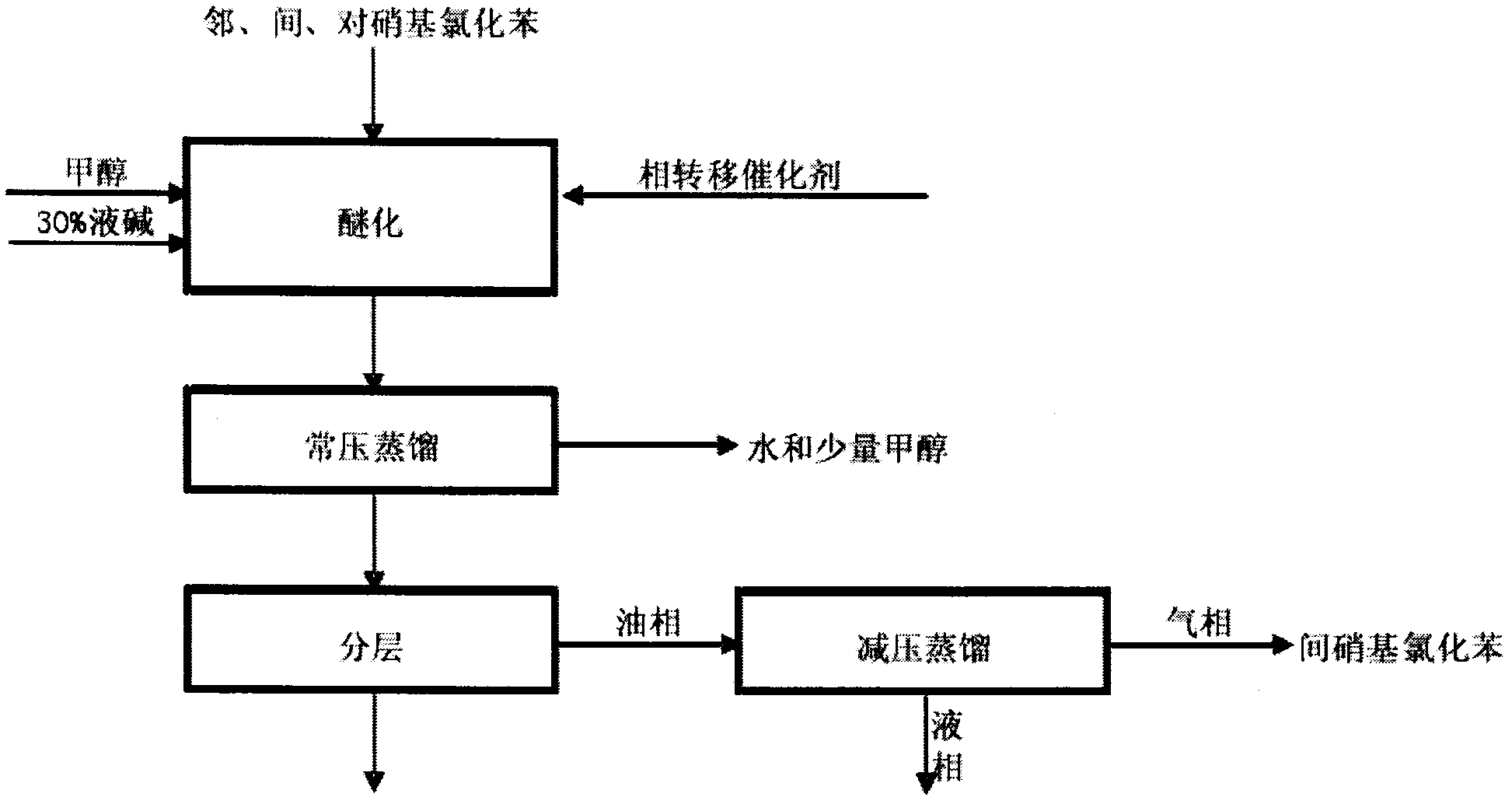
The invention relates to a method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene. According to the invention, mixed nitrochlorobenzene consisting of a product obtained after nitration of chlorobenzene, o-nitrochlorobenzene, m-nitrochlorobenzene and p-nitrochlorobenzene is used as a raw material, and in a methanol system, 30% of alkali lye and a phase-transfer catalyst are added for a reaction to prepare products of o-nitroanisole and p-nitroanisole, wherein m-nitrochlorobenzene does not participate in the reaction. The method provided by the invention overcomes the problems of great investment for rectifying tower equipment, great energy consumption in rectification, high cost, long process flow, harsh operation conditions, poor operational safety performances and generation of waste residues hardly to treat in rectification-crystallization separation of mixed nitrochlorobenzene in conventional production processes for o-nitroanisole and p-nitroanisole and overcomes the technical problems of generation of considerable alkaline waste water containing sodium hyposulfite and production of a poor-quality product in a sodium sulfide reduction process.
Owner:CHANGZHOU JIASEN CHEM
Method for synthetizing 3,5-dichloroaniline
InactiveCN103508901AReduce pollutionWith sustainable developmentOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneChlorobenzene
The invention discloses a method for synthetizing 3,5-dichloroaniline by using industrial production residues, namely meta-position oil, of p-nitrchlorobenzene and ortho-nitrochlorobenzene by two steps of chlorination and reductive dechlorination. The method adopts the following technical scheme: adding an organic solvent to nitrochlorobenzene meta-position oil as a raw material, directly producing a quintozene mixture by one-step chlorination under the effect of a catalyst, reacting for 0.5-10 hours under the condition of 10-180 DEG C, and then cooling to 30 DEG C; filtering out quintozene, and putting into an autoclave after washing into a neutral state by hot water; carrying out dechlorination reduction reaction under the effects of an organic solvent and the catalyst, wherein the reaction temperature is 60-200 DEG C, the reaction pressure is 1-20 MPa, and the reaction time is 1-20 hours; cooling to room temperature after the reaction is ended; leading in oxygen or air to stir after blowing off; filtering and distilling under reduced pressure to remove the solvent, so as to obtain the product 3,5-dichloroaniline, wherein the yield is 80-90%. Waste materials are changed into precious materials by a synthetic route adopted by the technology disclosed by the invention; the target of zero emission is achieved; the method has the advantages of sustainable development, energy conservation and consumption reduction, and small environmental pollution.
Owner:CHINA PETROLEUM & CHEM CORP +1
Continuous liquid-phase catalytic hydrogenation reduction method for production of o-chloroaniline
InactiveCN103360267AEnough time to stayShort processOrganic compound preparationChemical recyclingO-nitrochlorobenzeneHydrogenation reaction
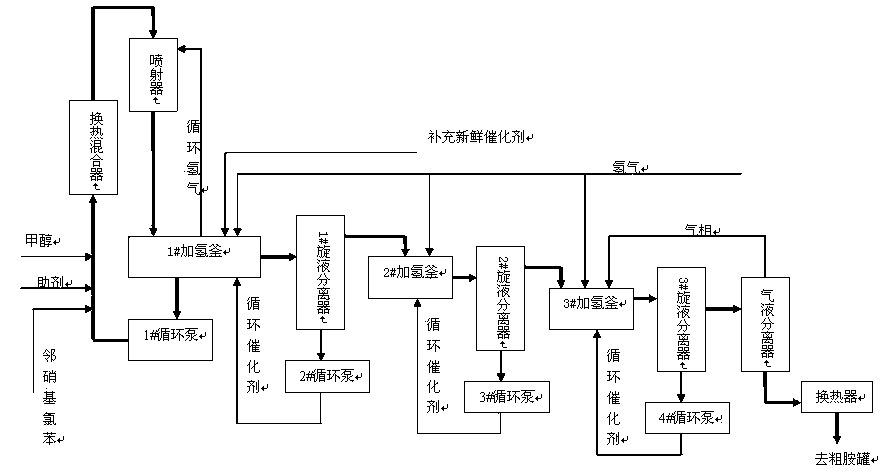
The invention discloses a continuous liquid-phase catalytic hydrogenation reduction method for production of o-chloroaniline. The method is characterized in that (1) multi-kettle cascade reaction is adopted during the hydrogenation reaction process, a No.1 hydrogenation kettle is provided with a heat exchange mixer and an injector, part of reaction materials circulate among the heat exchange mixer, the injector and the No.1 hydrogenation kettle through a circulating pump so as to fully convert o-nitrochlorobenzene to o-chloroaniline; and (2) a cyclone hydraulic separator is installed at the back of each hydrogenation reaction vessel to separate a catalyst in a hydrogenation liquid, and the separated catalyst returns to a corresponding hydrogenation kettle through the circulating pump for recycling. The process flow of the method is short; equipment used is simple; and defects of large investment of batch production, low utilization rate of equipment, large labor intensity, short service life of the catalyst, large loss of hydrogen and solvents, large fatigue strength of hydrogenation equipment and short service life are solved.
Owner:淮安嘉诚高新化工股份有限公司
Pt-load catalyst taking mesoporous carbon as carrier, as well as preparation method and usage thereof
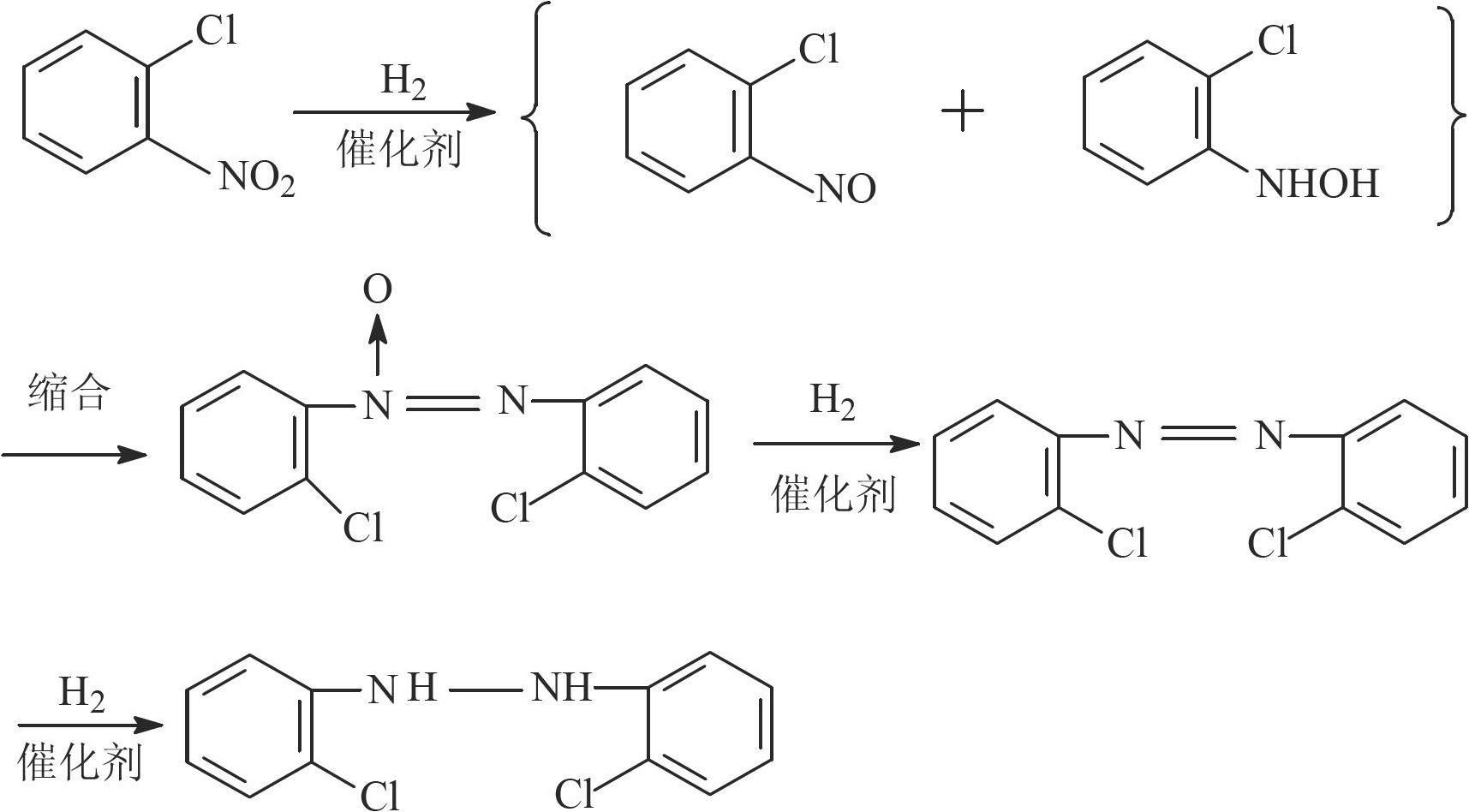
InactiveCN102671656AAdvantages of preparation methodHigh reactivityHydrazine preparationMetal/metal-oxides/metal-hydroxide catalystsO-nitrochlorobenzenePretreatment method
The invention relates to a Pt-load catalyst taking mesoporous carbon as a carrier, which is characterized in that the mesoporous carbon is pretreated before Pt is loaded; the pretreatment method comprises one in the following steps of: (1) adding the mesoporous carbon into a 10-30% hydrogen dioxide solution, soaking for 1-24hours, washing and removing hydrogen peroxide; (2) adding the mesoporous carbon into a 1-6M hydrochloric acid or nitric acid solution, soaking for 1-24hours, washing and removing hydrochloric acid or nitric acid; and (3) adding the mesoporous carbon into a 1-6M sodium hydroxide solution, soaking for 1-24hours, washing and removing alkali, wherein the load quantity of Pt is 0.5-5%. The invention also relates to the application of the Pt-load catalyst in preparation of 2, 2'-dichloro hydroazobenzene (DHB) from ortho-nitrochlorobenzene through hydrogenation. The catalyst uses the mesoporous carbon as the carrier, so that the internal diffusion resistance can be better reduced, and the reaction speed is accelerated. The Pt-load catalyst is high in reaction activity and selectivity, and can be repeatedly used for more than eight times, so that the using cost of the catalyst is lowered.
Owner:CHANGZHOU UNIV +1
Non-polluted method for producing o-chloroaniline with ferrous powder as reducer
InactiveCN101376634AReduce energy consumptionReduce productionOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneIron powder
The invention relates to an environment friendly method for reduction producing o-chloroaniline with iron powder. The method comprises adopting o-nitrochlorobenzene as the raw material and the iron powder as a reducing agent; separating o-chloroaniline, water andiron mud by vacuum distilling to obtain a coarse o-chloroaniline product; and refining by rectifying to obtain o-chloroaniline. The method solves the defects in the prior art on heavy pollution and high energy consumption, and implements the clear production of o-chloroaniline.
Owner:淮安嘉诚高新化工股份有限公司
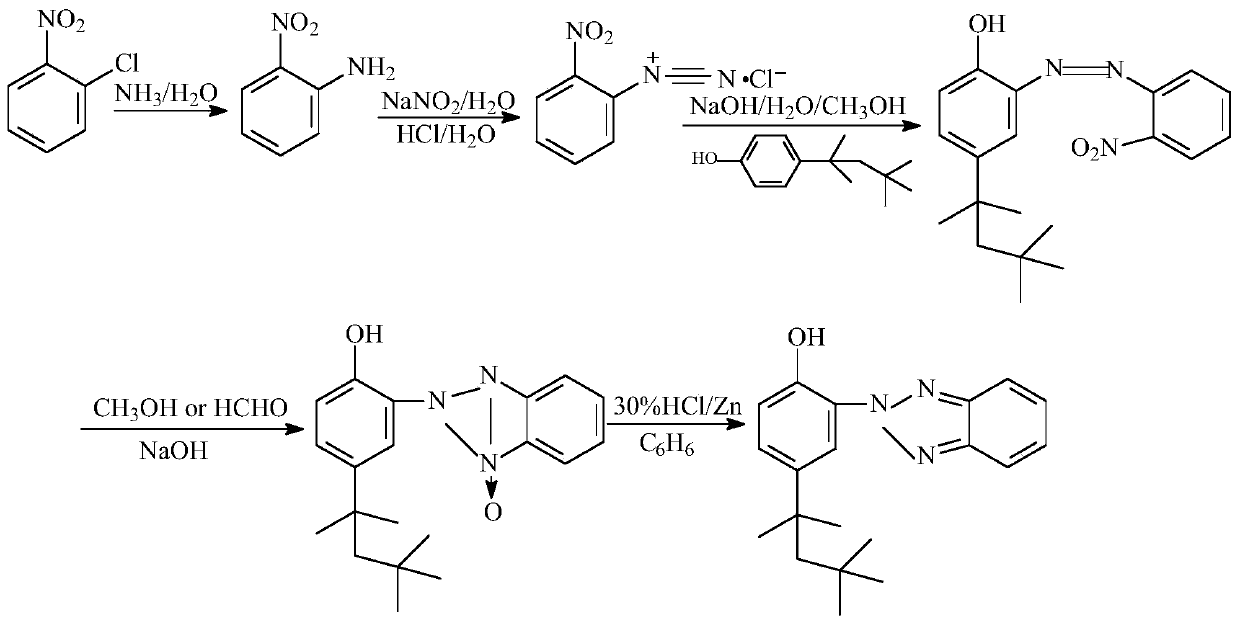
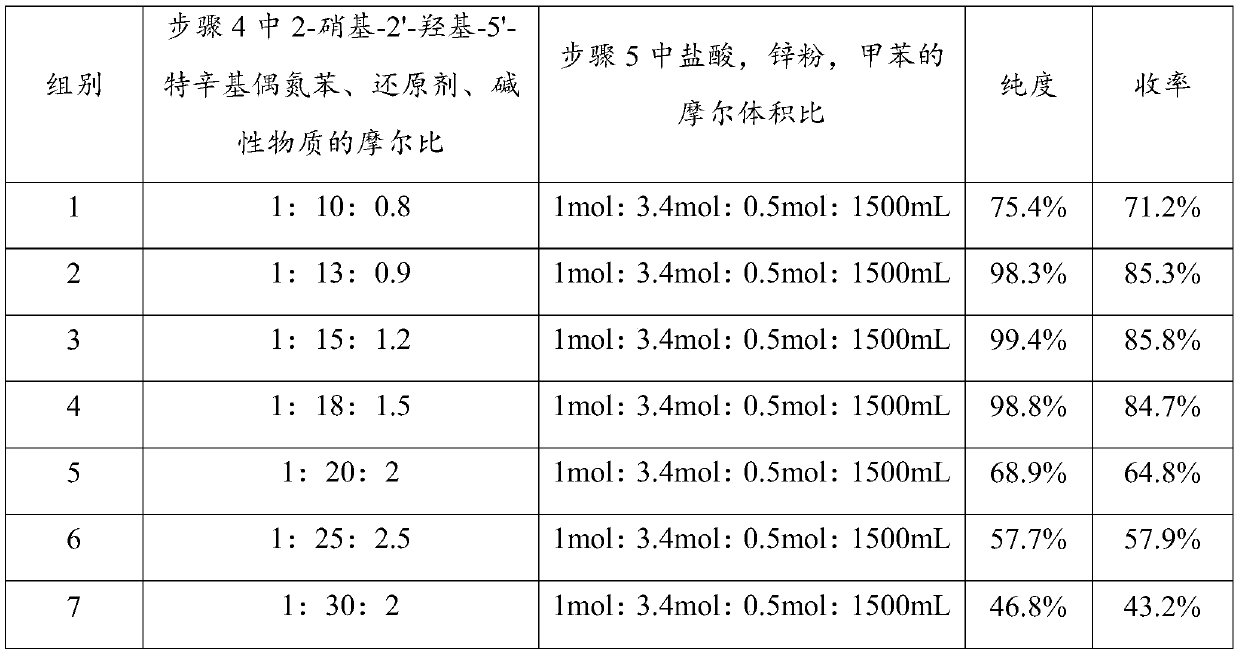
Preparing method of ultraviolet absorber UV-329
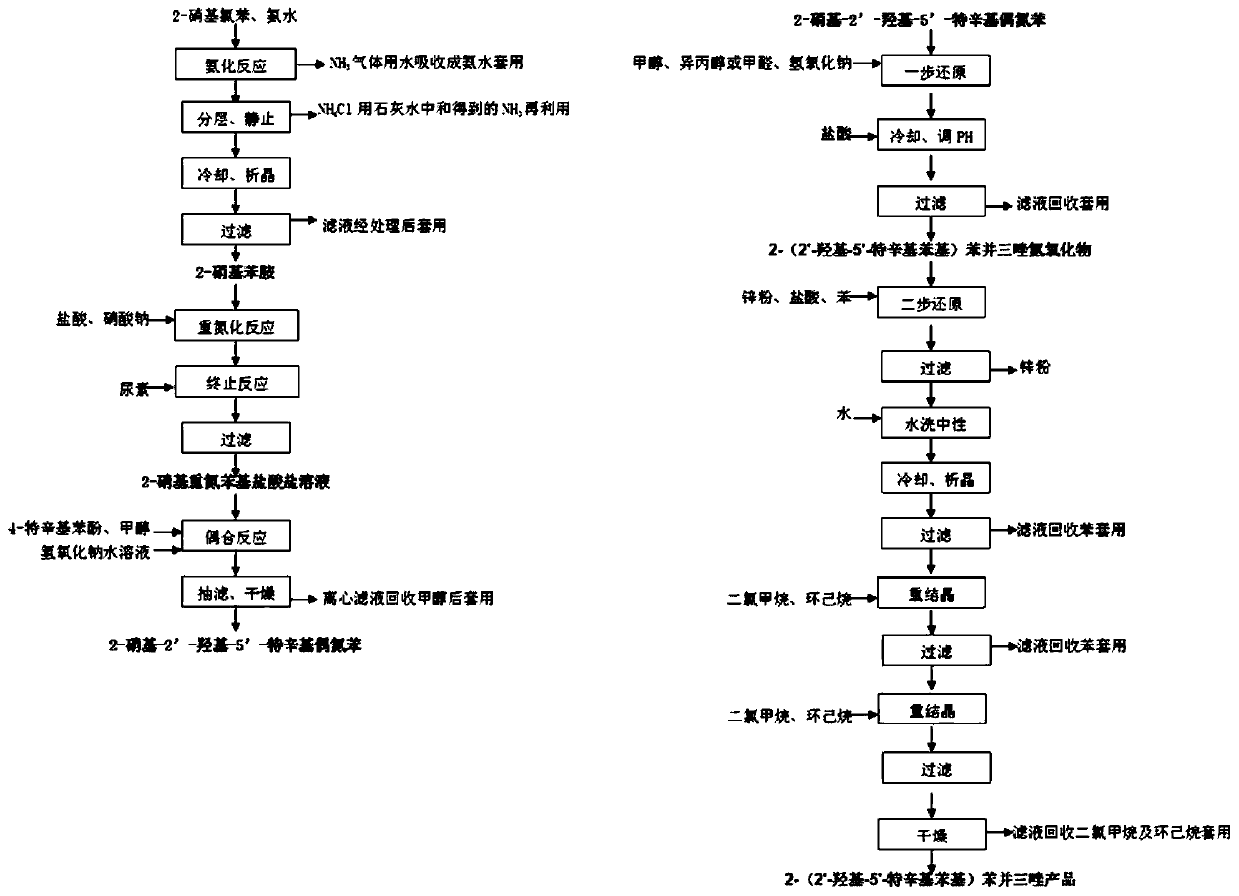
The invention provides a preparing method of an ultraviolet absorber UV-329. The preparing method comprises the following steps of conducting ammoniation, diazotization, coupling, first-step reduction, second-step reduction reaction and post-treatment on ortho-nitrochlorobenzene to prepare a 2-(2'-hydroxy-5'-octylphenyl)benzotriazole product. According to the preparing method, ortho-nitrochlorobenzene is ammonified by adopting ammonium hydroxide with the concentration of 10-25%, then is subjected to diazotization with nitrite to produce diazonium salt, no diazotization needs to occur under a strong acid medium, by-product ammonium hydroxide of existing products of a company is comprehensively utilized, low emission of three wastes is achieved, and the required cost is low; a two-step reduction method is adopted in a reduction ring closing process of intermediate azo products, the situation is avoided that a strong reduction agent directly reduces azo double bonds into amine, impurity production is reduced, and not only is the purity of the products increased but also the product yield is increased.
Owner:XIANGYANG JINDACHENG FINE CHEM
Method for producing chloroethylene by homogeneous mercury-free catalysis
InactiveCN104926592AReduce dosageSimple processPreparation by halogen halide additionO-nitrochlorobenzeneO-chlorotoluene
The invention provides a method for producing chloroethylene by homogeneous mercury-free catalysis. According to the method, acetylene and hydrogen chloride are taken as raw materials and chloroethylene is generated under the effect of a homogeneous catalyst. One or more of PdCl2, AuCl3, FeCl3, ZnCl2 and CuCl2 are taken as catalysts, one or more of NH4Cl, NaCl, KCl, BaCl2, MgCl2 and CaCl2 are taken as cocatalysts, o-nitrochlorobenzene, o-chlorotoluene or o-dichlorobenzene is taken as a solvent, and a homogeneous catalysis system is formed. Under the reaction conditions of normal pressure and temperature of 130 DEG C, the acetylene conversion rate and the chloroethylene selectivity can reach about 95% and more than 95%, and the catalyst life is more than 3,000 hours. The method adopts the nontoxic and mercury-free homogeneous catalyst for producing the chloroethylene, so that a supported catalyst for producing chloroethylene by hydrochlorination of acetylene in existing production is avoided, the problems of easiness for generating carbon deposit sintering and the like in a conventional process carrier are avoided, and homogeneous production of the chloroethylene is realized.
Owner:YANGZHOU UNIV
Reclaiming technique by using resin to adsorb nitro chlorobenzene in wastewater from producing nitro chlorobenzene
InactiveCN1562789AReduce contentSolid sorbent liquid separationWater/sewage treatment by sorptionLiquid wasteO-nitrochlorobenzene
The method is to to get rid of mechanical impurity in prodn, waste water of nitrochlorobenzene by pretreatment, then to make it passing through adsorption column filled by styrene-divinylbenzene absorbing resin in flow quantity less than 10 BV / h, the nitrochlorobenzene is selected absorbed on the resin, nitroxylene is not absorbed, quantity of absorbed nitrochlorobenzene is less than 2 mg / L, the resin absorbing nitrochlorobenzene is deadsorbed and regenerated by using water vapour as deadsorpting agent, the generated gas-liquid mixture is cooled and separate to recover nitrochlorobenzene. More than 99 percent of o-nitrochlorobenzene and para-nitrochlorobenzene can be recovered from waste water by this invention. The recovered matter can be send back to recti-ficating production process to be rectificated separated to obtain o-nitrochlorobenzene and para-nitrochlorobenzene products.
Owner:NANJING UNIV
Material layering process for reduced o-phenylenediamine
ActiveCN105017027AImprove qualityHigh recovery rateAmino compound purification/separationOrganic compound preparationO-nitrochlorobenzeneMan-hour
The present invention provides a material layering process for reduced o-phenylenediamine. The process comprises: firstly, mixing o-nitrochlorobenzene and ammonia to acquire o-nitroaniline; then adding sodium sulphide to the o-nitroaniline, wherein after reduction, a reduction pot contains a hydrate of a mother solution, o-phenylenediamine and a salt; and separating the o-phenylenediamine by using a two-stage separation technique. The present invention employs the self-developed technique for layering reduced o-phenylenediamine, thereby reducing the energy consumption, shortening the man-hour, reducing the rectification residues, providing the necessary condition for subsequent smooth rectification, and acquiring the o-phenylenediamine with high quality; providing a basis for increasing productivity of carbendazim; further improving the product quality of the o-phenylenediamine and the recovery rate in production process, reducing unit consumption, and reducing costs;and improving the operating environment and greatly reducing the labor intensity.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Yellow peach planting method
InactiveCN106171758AImprove survival rateFast growthMagnesium fertilisersAlkali orthophosphate fertiliserDiseaseO-nitrochlorobenzene
The invention relates to a yellow peach planting method, and relates to the technical field of yellow peach planting. The yellow peach planting method includes the following steps of selecting soil; deeply digging a tree pit; treating a sapling, wherein a trunk is wrapped with a layer of gauze, the tightness degree is suitable when the thumb can be inserted into the portion between the gauze and the trunk, the outer side is wrapped by a plastic film, the gauze is soaked in a soaking solution first, the soaking solution is prepared from, by weight, 1-3 parts of water-soluble ammonium polyphosphate, 1-3 parts of fluosilicic acid, 5-8 parts of bentonite, 4-8 parts of gelatin, 1-3 parts of o-nitrochlorobenzene, 0.1-0.3 part of dimethyl sulfone, 0.2-0.5 part of dimethylformamide and 100-200 parts of water, the gauze and the root are soaked together for 4-6 h, and then the sapling is planted; management after planting, wherein the sapling is watered once at the interval of 15-20 days, and water is injected into the gauze from the upper side of the trunk wrapped by the gauze during each time of watering. The method is high in survival rate and growth speed, and the tree hardly suffers from diseases and insects, which is beneficial for increasing the planting yield.
Owner:全椒县管坝民族玉龙生态农业发展有限公司
Device and method for production of o-chloroaniline through continuous catalytic hydrogenation reduction of o-nitro chloro benzene
InactiveCN104098477ACycle fastImprove mass transfer effectOrganic compound preparationAmino compound preparationO-nitrochlorobenzenePartial hydrogenation
The invention discloses a device and method for production of o-chloroaniline through continuous catalytic hydrogenation reduction of o-nitro chloro benzene. The method comprises the following steps: o-nitro chloro benzene, a catalyst and a dechlorination inhibitor are put into a hydrogenation reactor, and bottom materials are distributed well; nitrogen and hydrogen are sequentially used for displacing a system completely; a feed valve is opened, the o-nitro chloro benzene and the dechlorination inhibitor are continuously fed into the hydrogenation reactor, wherein the mass ratio of the o-nitro chloro benzene to the catalyst to the dechlorination inhibitor is 1:(0.001-0.005):(0.001-0.005); hydrogen is fed into the hydrogenation reactor for hydrogenation reaction, wherein the reaction temperature is 60-100 DEG C and the pressure is 0.5-1.5 MPa; hydrogenated liquid enters a heat exchanger firstly through a circulating pump and then passes through a cross-flow filter; the catalyst and part of hydrogenated liquid as well as fresh raw materials together enter an ejector for circulation, and the other part of the hydrogenated liquid is extracted out continuously, and o-chloroaniline is obtained after treatment. The device and the method disclosed by the invention have advantages that the production cycle is short, the catalyst selectivity is high, the dechlorination rate is low, the yield is high, the quality is stable, the equipment efficiency is high and continuous production is realized.
Owner:淮安嘉诚高新化工股份有限公司
Method for treating o-nitrophenol production wastewater
InactiveCN110980864ANo secondary pollutionRecycling without distillationWater treatment parameter controlOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
The invention belongs to the field of chemical wastewater treatment and particularly relates to a method for treating o-nitrophenol production wastewater. For wastewater produced after o-nitrochlorobenzene is hydrolyzed to prepare o-nitrophenol, ethanol is adopted as a desorbent in the method; and under the premise of utilizing existing process equipment and basis procedures, valuable o-nitrophenol can be effectively recovered without the need of toxic / expensive chemical agents, special treatment equipment or extra energy consumption. On this basis, the method can achieve effective utilizationof an o-nitrophenol recovery product, improve the overall process efficiency for preparing the o-aminophenol and the recovery efficiency of salts and other by-products, and avoid secondary pollution.The invention further provides a method for producing o-aminophenol related to the aforementioned method for treating wastewater.
Owner:HUBEI HONGXIN CHEM CO LTD
Process of producing nitrobenzether aminobenzether amidobenzether from chlorobenzene
InactiveCN100494159CReduce manufacturing costImprove production stabilityOrganic compound preparationCarboxylic acid amides preparationO-nitrochlorobenzeneChloride salt
Owner:CHANGZHOU JIASEN CHEM +1
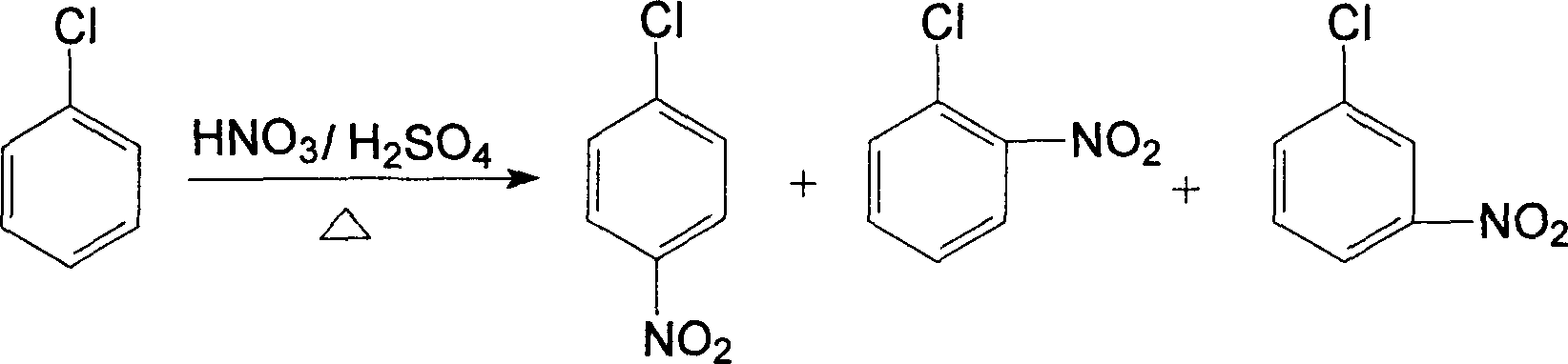
Location nitration process for chlorobenzene
InactiveCN1634855AEasy to recycleReduce the contrast ratioNitro compound preparationO-nitrochlorobenzeneRare-earth element
Chlorobenzene located nitrification method is a method for preparing nitro chlorobenzene of an intermediate in fine chemical industry, especially a method for improving the yield of o-nitro chlorobenzene by using water soluble Lewis acid to catalytically nitrifying chlorobenzene. The provided method comprises: nitric acid, chlorobenzene and water soluble Lewis acid catalyst are put into the 1,2-dichloroethane solvent to react at the temperature of 30C. to 100C. with the mol ratio of 1-3í†1í†0.01-0.15, and the reaction time is 3-30 hours, after cooled to room temperature, the mixture is washed by water, extracted by chloroform, dried in the rotary evaporator, separated by chromatographic column to obtain yellow oily liquid. The water soluble Lewis acid catalyst has the molecular formula as follows: Ln(OSO2CF3)3, wherein Ln is Sc or Y or La family rear earth element, or M(OSO2CF3)4, wherein M is Hf or Zr.
Owner:SOUTHEAST UNIV
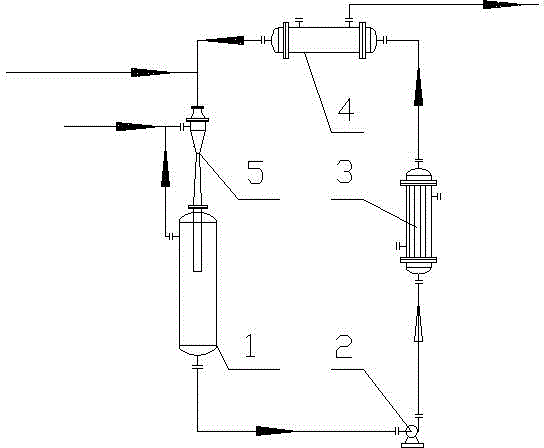
Rectification and separation device and method
The invention discloses a rectification and separation device and method. The rectification and separation device and method are used for separating three isomers, namely m-nitrochlorobenzene, p-nitrochlorobenzene and o-dichlorobenzene. According to the device and the method, a rectifying tower is separated by a partition board arranged in the vertical direction, and majorized technology parameters are adopted, so that using one rectifying tower to complete whole separation of the m-nitrochlorobenzene, p-nitrochlorobenzene and o-dichlorobenzene is realized. Therefore, the rectification and separation device and method provided by the invention can reduce energy consumption, equipment investment and occupied area.
Owner:扬州通扬化工设备有限公司
Method for synthesizing o-aminoanisole by hydrogenation method
InactiveCN109053472AIncrease contact areaHigh purityOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneSodium methoxide
The invention discloses a method for synthesizing o-aminoanisole by a hydrogenation method. The synthesis method specifically comprises the following steps: adding metallic sodium to excess methanol to prepare a methanol solution of sodium methoxide, and then spraying o-nitrochlorobenzene and the methanol solution of sodium methoxide into an etherification kettle; firstly, performing centrifugal separation, then transferring to a distillation kettle for distillation, and then crystallizing and filtering; firstly, introducing the hydrogen gas to exhaust the gas, atomizing o-nitroanisole, usinga catalyst for catalyzing the reaction, introducing nitrogen gas to the kettle to exhaust the gas after the reaction is completed, then cooling and crystallizing, centrifuging at low temperature for separation and filtering, and repeatedly operating for 2 to 3 times to obtain o-aminoanisole. The method for synthesizing o-aminoanisole by the hydrogenation method uses nitrogen-doped porous carbon asa carrier for the catalyst, and the catalyst is made into a lattice. Contact area of the reactant is large, and the reaction proceeds rapidly. A methoxy reagent is directly prepared from the metallicsodium and methanol, and the methoxy reagent is dissolved with methanol to prepare a solution for spraying, so as to accelerate the reaction and promote the reaction to proceed forward.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Continuous ammoniation method of aniline organic intermediates
InactiveCN107382747ALower synthesis costHigh yieldOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneOrtho-nitroaniline
The invention discloses a continuous ammoniation method of aniline organic intermediates. The continuous ammoniation method concretely comprises the following steps of feeding o-nitrochlorobenzene and concentrated ammonia liquid into a six-stage serially connected high-pressure autoclave in a continuous feeding and continuous discharging mode; performing reaction for 10 to 15 hours at the inside pressure of 5.3MPa and the reaction temperature being 170 DEG C; obtaining ortho-nitroaniline; then, transferring the reaction liquid into a secondary reaction kettle; adding caustic alkali for regulating the pH value to be 12 to 13; then, performing reaction for 10 to 30min; obtaining high-purity ortho-nitroaniline and byproducts of ammonium chloride. The continuous ammoniation method has advantages that useful substances can be effectively recovered from intermediates of ortho-nitroaniline produced from carbendazim after the primary reaction is completed; the content of the ortho-nitroaniline after the hydrogenation is 98 percent or higher; the synthesis cost is reduced; the economic benefits are improved; the ammoniation yield is improved; the continuous ammoniation method and process design of the aniline organic intermediates are optimized; the continuous ammoniation method and process design requirements of the aniline organic intermediates are met.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Synthesis method of o-amino pheylmethyl ether
InactiveCN104086448AEasy to useEasy to prepareOrganic compound preparationAmino-hyroxy compound preparationChemical industryO-nitrochlorobenzene
The invention discloses a preparation method of o-amino pheylmethyl ether, and relates to the technical field of chemical industry. The method comprises the following steps: adding o-chloronitrobenzene, methanol and a 40-percent sodium hydroxide solution into a high-pressure reaction kettle in sequence, raising the temperature in the kettle to 40 DEG C, and stirring; raising the temperature to 85 DEG C, controlling the pressure at 0.28-0.32MPa, reacting for 8 hours, distilling, removing an internal methanol solution, adding hot water of 70 DEG C for washing, standing for delaminating, and performing liquid separation to obtain o-nitroanisole for later use; putting o-nitroanisole into the high-pressure reaction kettle, adding a sodium sulfide aqueous solution, controlling the pressure at 0.05MPa, controlling the temperature at 118-120 DEG C, pressurizing, refluxing, cooling to 50-60 DEG C, preserving heat for 5 hours, performing liquid separation, removing internal waste water, distilling, crystalizing, drying to obtain finished o-amino pheylmethyl ether, packaging and warehousing. The preparation method has the beneficial effects of convenience and easiness in preparation, environmental friendliness, pollution freeness, ready availability of raw materials, small equipment investment, high purity and convenience in operation. The prepared o-amino pheylmethyl ether has a good use effect, and is safe and reliable.
Owner:安徽佑骏商品混凝土有限公司
Preparation method of 1,2-di(2-aminophenoxy)ethane
ActiveCN105669470AHigh purityHigh reaction yieldOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneChloride
The invention discloses a preparation method of 1,2-di(2-aminophenoxy)ethane. According to the method, o-nitrochlorobenzene which is used as a starting material and glycol firstly undergo a condensation reaction under the alkaline condition and in the presence of a phase transfer catalyst so as to prepare 1,2-di(2-nitrophenoxy)ethane; and then, the 1,2-di(2-nitrophenoxy)ethane undergoes catalytic hydrogenation so as to prepare 1,2-di(2-aminophenoxy)ethane. The phase transfer catalyst is triethylbenzylammonium chloride. molar ratio of o-nitrochlorobenzene to glycol is 2.2:1-3.0:1. By selecting the proper phase transfer catalyst TEBAC and using excess o-nitrochlorobenzene in the condensation reaction, high-purity 1,2-di(2-aminophenoxy)ethane can be obtained. Especially, yield of the condensation reaction can reach 95% and above. Thus, the preparation method is suitable for industrial mass production.
Owner:CHANGZHOU YONGHE FINE CHEM
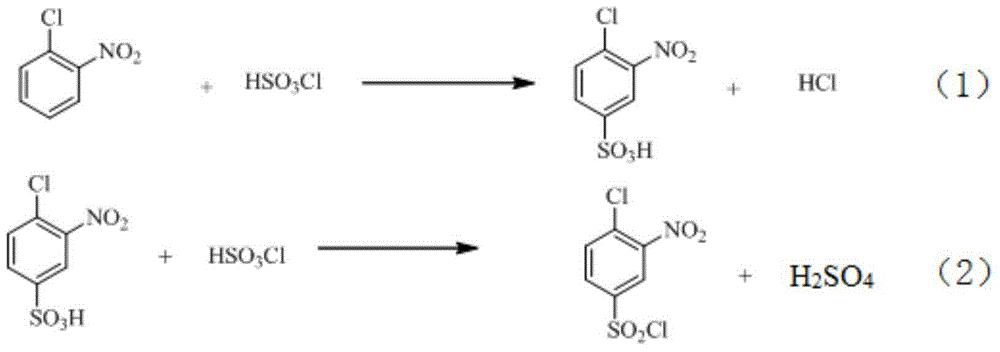
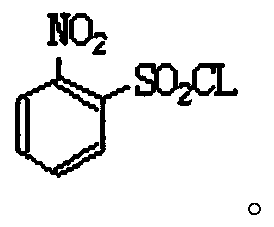
Industrial production method of o-nitrobenzenesulfonyl chloride
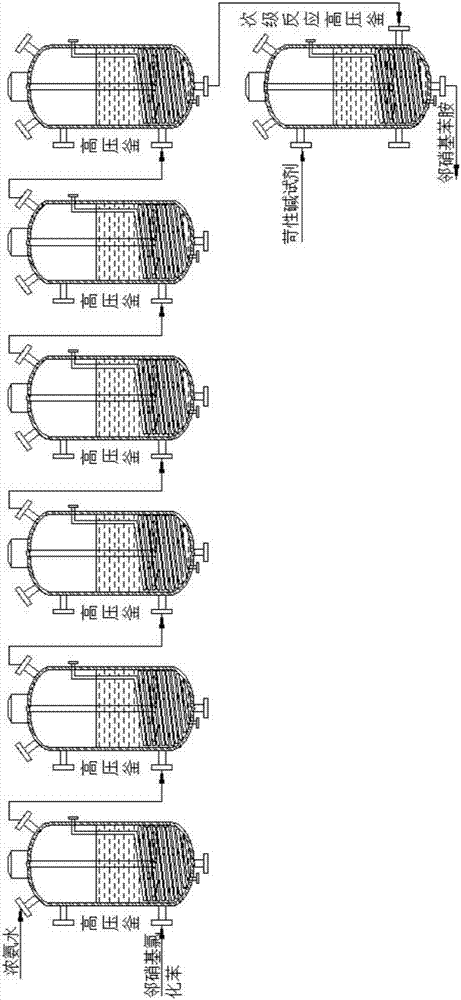
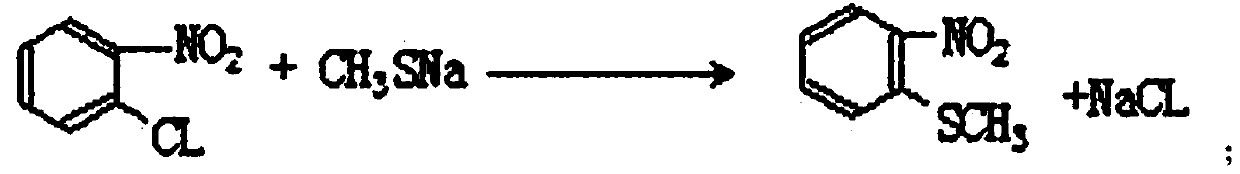
InactiveCN110105251ALow toxicityAddressing Purity IssuesOrganic compound preparationSulfonic acid preparationO-nitrochlorobenzeneNitrobenzene
The invention discloses an industrial production method of o-nitrobenzenesulfonyl chloride. The method comprises the following steps that o-nitrochlorobenzene and sodium methyl mercaptide are subjected to an etherification reaction, filtering is conducted, an obtained filter cake is subjected to recrystallization, and through centrifugation separation and drying, a dry product of o-nitrobenzene dimethyl sulfide is obtained; the dry product of o-nitrobenzene dimethyl sulfide is subjected to a chlorination reaction in batches to obtain a wet crude product, an appropriate amount of hydrochloric acid is added in a chlorination reaction system, the chlorination reaction is carried out in a hydrophilic organic acid solvent, and the mole ratio of the o-nitrobenzene dimethyl sulfide to water during the chlorination reaction is 1:(5-15); a finished product of o-nitrobenzenesulfonyl chloride is obtained through refining and drying. Through HPLC detection, the content of the o-nitrobenzenesulfonyl chloride synthesized by means of the method is 98-98.5%; the yield is 0.72-0.75, the acquisition rate is 0.97 or above, the turbidity (ppm) is 1.5-2, and the melting point is 66-67 DEG C. By adopting the hydrophilic organic acid solvent, the problems about large-scale production discharging, yield and quality are solved, and meanwhile the purposes of mixed application and post-treatment separation of large-scale production water-soluble solvents are achieved.
Owner:苏州市泽宸贸易有限公司
Preparation method for light hydrocarbon copper sheet corrosion inhibitor
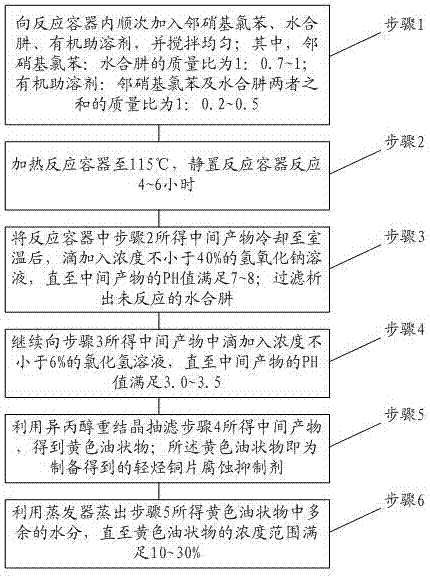
PendingCN106995385AAvoid corrosionGood oil solubilityHydrazine preparationO-nitrochlorobenzeneFiltration
The invention relates to the technical field of light hydrocarbon corrosion inhibition, in particular to a preparation method for a light hydrocarbon copper sheet corrosion inhibitor. The inhibitor prepared by the preparation method can effectively lower the corrosiveness of a light hydrocarbon copper sheet, thereby achieving the technical effect of stabilizing the corrosiveness of the light hydrocarbon copper sheet. The preparation method for the light hydrocarbon copper sheet corrosion inhibitor comprises the following steps: adding o-nitrochlorobenzene, hydrazine hydrate and an organic cosolvent into a reaction container in sequence; heating, standing and reacting; performing alkali treatment for precipitating hydrazine hydrate; performing acid treatment for adjusting the PH value; recrystallizing with isopropanol, and performing suction filtration.
Owner:CHINA PETROLEUM & CHEM CORP +1
Anti-ultraviolet-aging emulsified asphalt and preparation method thereof
PendingCN112778781AImprove conductivityHigh resistivityBuilding insulationsO-nitrochlorobenzenePolymer science
The invention discloses anti-ultraviolet-aging emulsified asphalt and a preparation method thereof. The method comprises the following steps: S1, preparing a mixed solution of hydrochloric acid and sodium nitrite, adding o-nitrochloroaniline and uric acid, and conducting stirring to obtain diazonium salt; S2, placing the diazonium salt in an ethanol solution, conducting stirring and dispersing, adjusting the pH value, conducting stirring, and adding hydroquinone to obtain a material A; S3, adding thiourea dioxide into the material A, and conducting stirring to obtain a copolymer monomer; and S4, adding aniline into a hydrochloric acid solution, conducting stirring, sequentially adding an emulsifier, ammonium persulfate, a flexibilizer, a curing agent and water-borne epoxy resin, adding the copolymer monomer and matrix asphalt, and conducting emulsifying to obtain the emulsified asphalt. The prepared emulsified asphalt mixture is good in conductivity, high in stability, excellent in ultraviolet aging resistance, mild in technological process, simple in reaction principle, very suitable for industrial production and market application and popularization, capable of greatly prolonging the service life of an asphalt material and extremely high in economic value and practicability.
Owner:胡浩
Production method for improving purity of o-nitrochlorobenzene
InactiveCN110294677AAchieve separationHigh purityOrganic compound preparationNitro compound preparationO-nitrochlorobenzeneChlorobenzene
The invention discloses a production method for improving the purity of o-nitrochlorobenzene. The production method comprises the following steps that anhydrous chlorobenzene, a sulfuric acid solutionand a nitric acid solution are added into a nitrating kettle for a reaction until the amounts of substances do not change anymore; an obtained nitrochlorobenzene mixture is neutralized, filtered andwashed to obtain a precipitant; the washed nitrochlorobenzene mixture and water are heated and then enter an evaporation chamber to achieve azeotropy of the mixture and water, an azeotrope enters a separation chamber, then cooling is conducted to 10-30 DEG C, and filtering is conduced to obtain a crude product of o-nitrochlorobenzene; the crude product is transferred into a washing kettle to be washed and then dried, and then the high-purity o-nitrochlorobenzene is obtained. According to the production method, the nitrochlorobenzene mixture and water are subjected to flashing evaporation in the evaporation chamber, and separation is conducted to obtain the high-purity o-nitrochlorobenzene. According to the production method, an azeotropy separation process is adopted for avoiding the crystallization and heating process, the energy consumption can be effectively lowered, the purity of the product is improved at the same time, complete separation of three kinds of nitrochlorobenzene is achieved, the content of the product o-nitrochlorobenzene reaches 99% or above, and the yield reaches 98% or above.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com