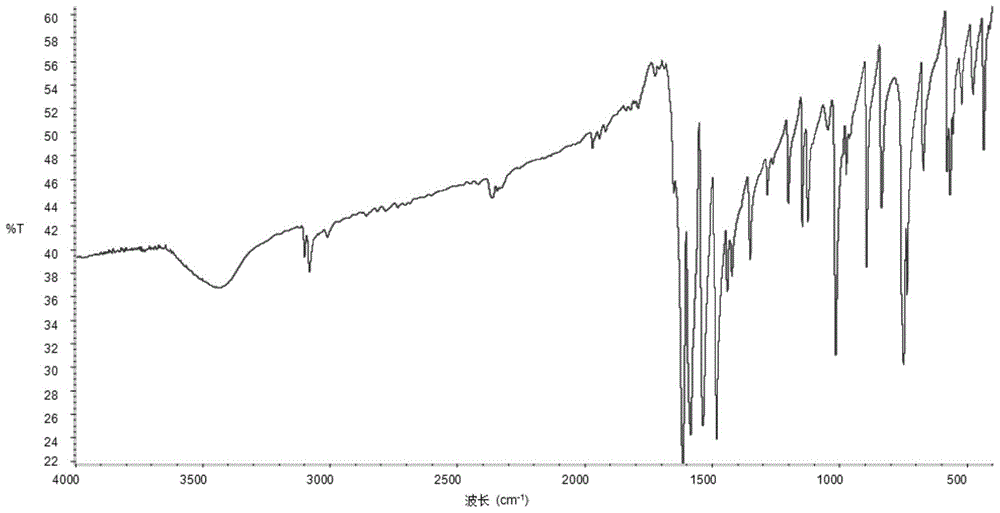
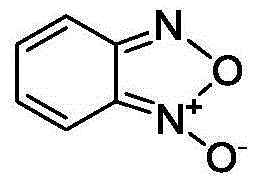
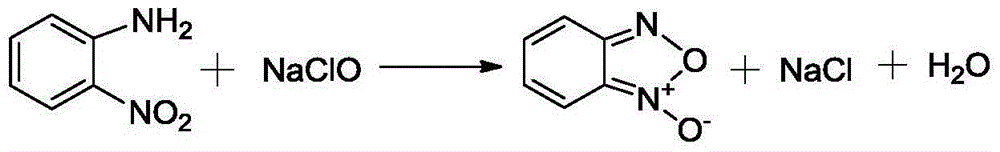
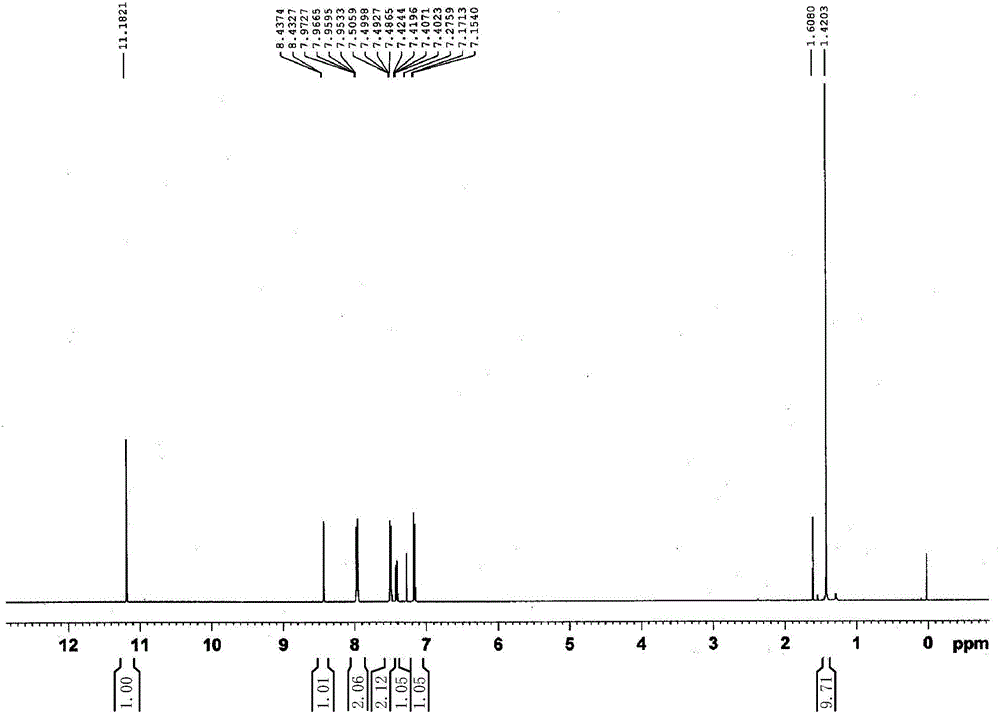
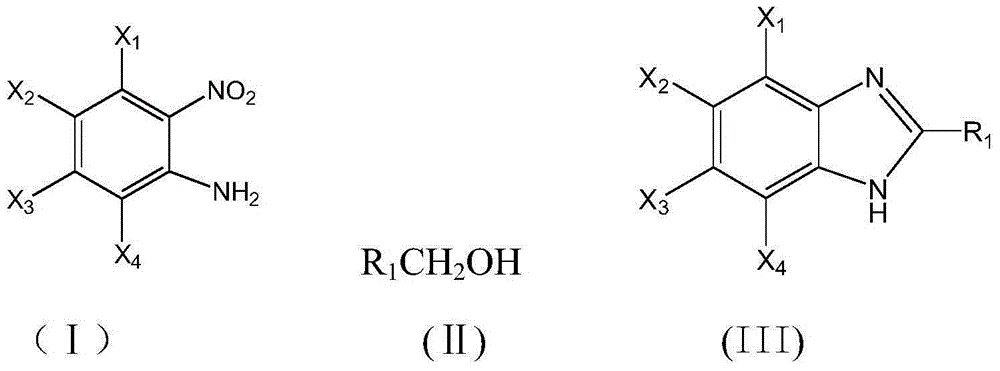Patents
Literature
39 results about "Ortho-nitroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
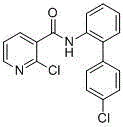
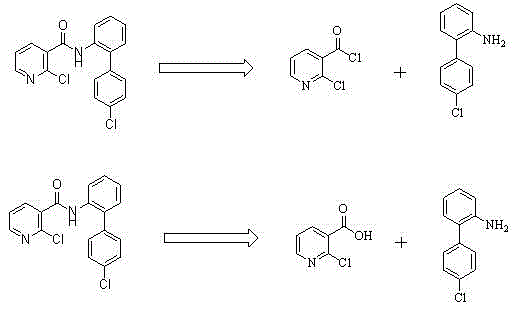
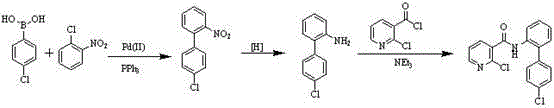
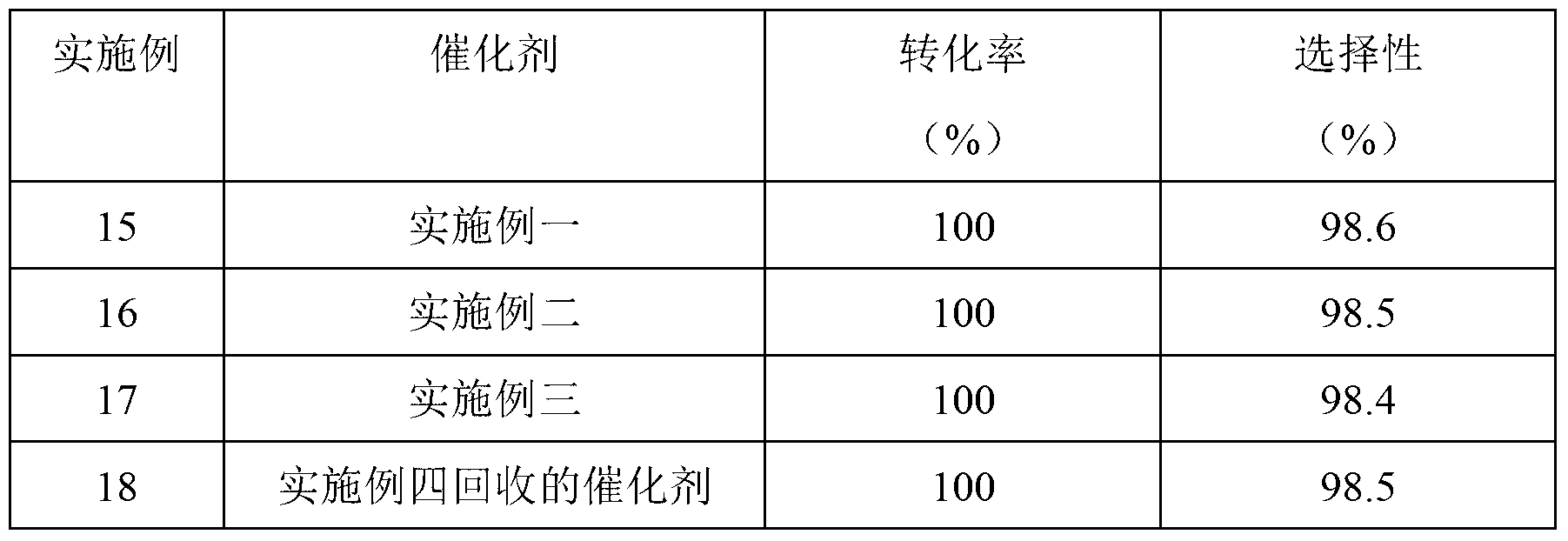
Preparation method of nicotinamide fungicide namely boscalid
The invention discloses a preparation method of a nicotinamide fungicide namely boscalid. The preparation method comprises the following steps: starting from a raw material namely para chlorobromobenzene, performing Grignard reaction, and synthesizing p-chlorobenzeneboronic acid; starting from ortho-nitroaniline, synthesizing o-nitro halogenated benzene, or directly synthesizing o-amino halogenated benzene; and under the action of specific catalysts, enabling p-chlorophenylboronic acid to perform a series of reactions such as coupling, reduction, amidation and the like with o-nitro halogenated benzene or o-amino halogenated benzene to prepare boscalid. The preparation method disclosed by the invention is simple to operate, high in yield, small in pollution, low in cost and high in purity, and is very suitable for industrial production.
Owner:SUZHOU TOKIND CHEM CO LTD
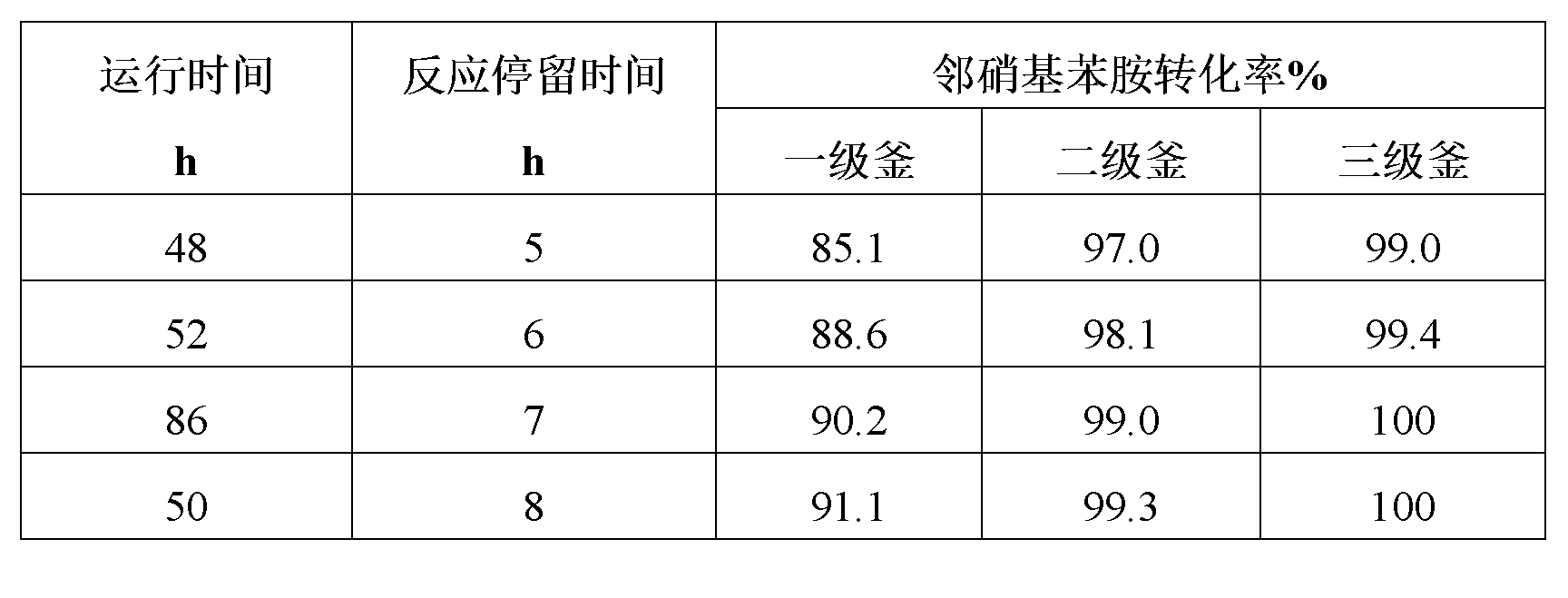
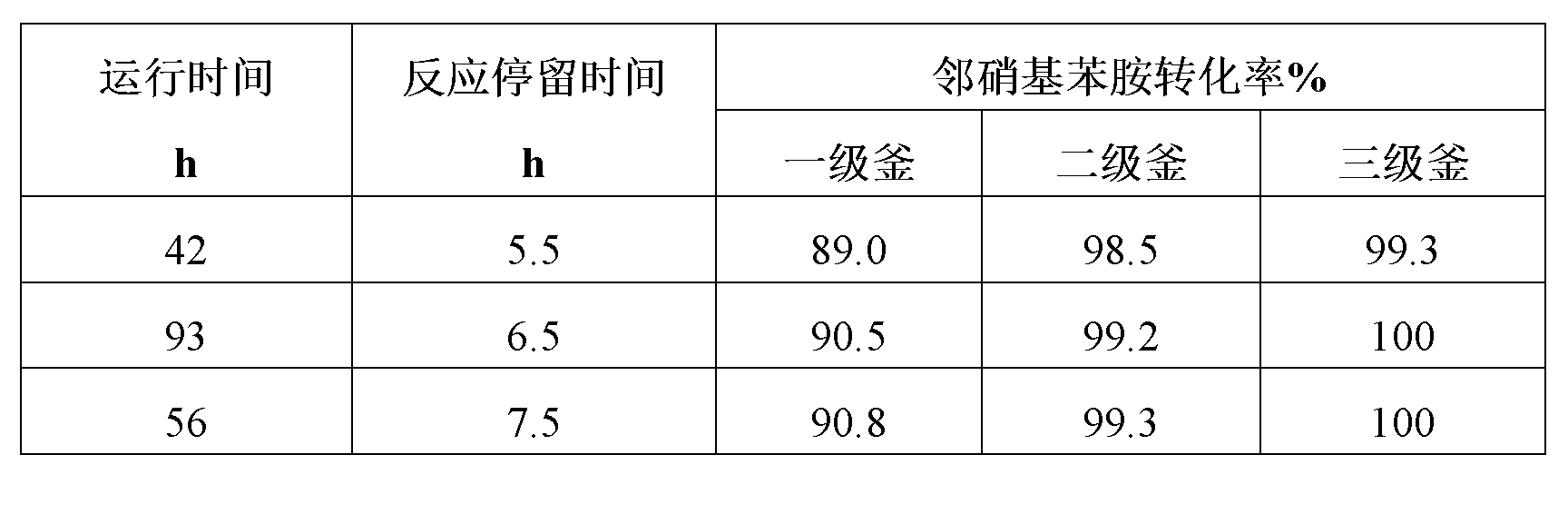
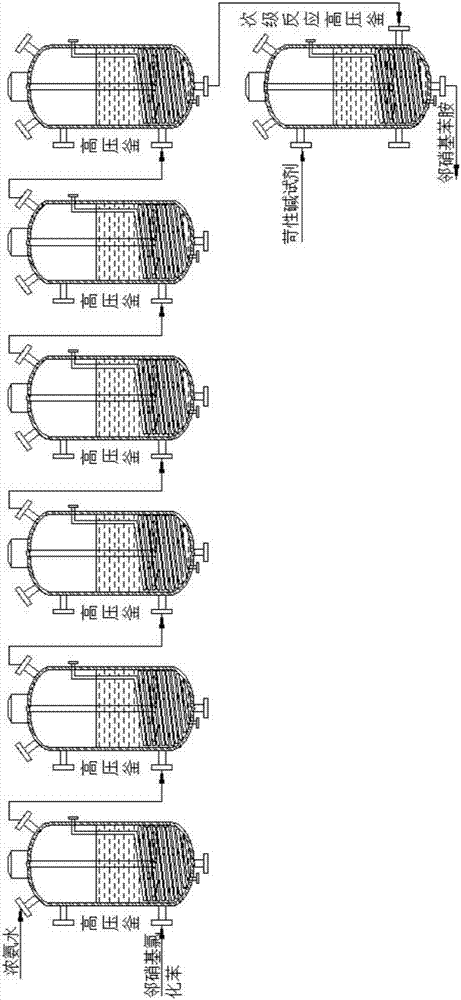
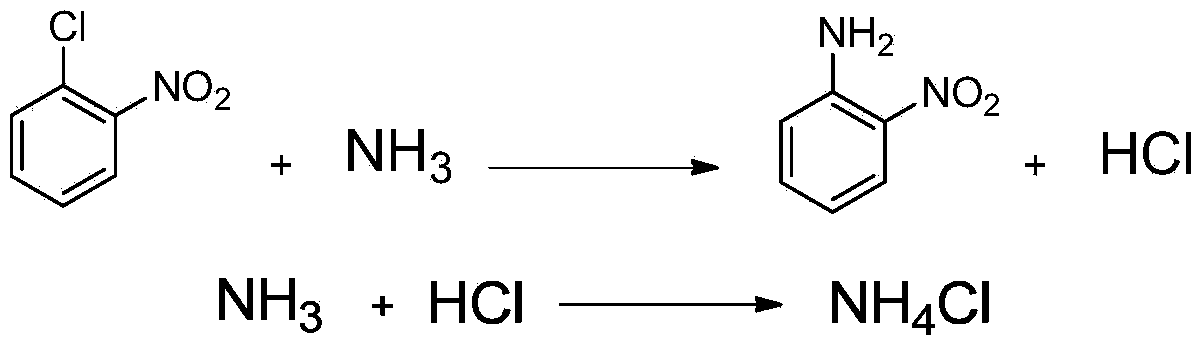
Method for preparing ortho-nitroaniline by kettle-type continuous operation
InactiveCN101602676AGood effectIncrease production capacityAmino preparation by hydrogen substitutionOrtho-nitroanilineOil water
The invention relates to a method for preparing ortho-nitroaniline by kettle-type continuous operation, belonging to the organic chemical engineering field; pressure-resistant reaction kettles are sequentially connected in series, and high-order differences are formed among the reaction kettles, so as to lead the reaction material to overflow to the next-level reaction kettle; 0-nitrophenyl and ammonia water are added in a first-level reaction kettle in the pressure-resistant reaction kettle, and the mixture is reacted under the condition that the temperature in the kettle is 150 DEG C to 250 DEG C, and pressure is 3MPa to 10MPa, and then the reacted products are oil-water laminated; after water is cooled, the ortho-nitroaniline is dissolved out in a solid way and is press-filtered into filter cakes, namely, the ortho-nitroaniline is obtained. The method has simple flow, stable yield coefficient of products and less three wastes, and is easy to realize industrialization.
Owner:JIANGSU YANGNONG CHEM GROUP +1
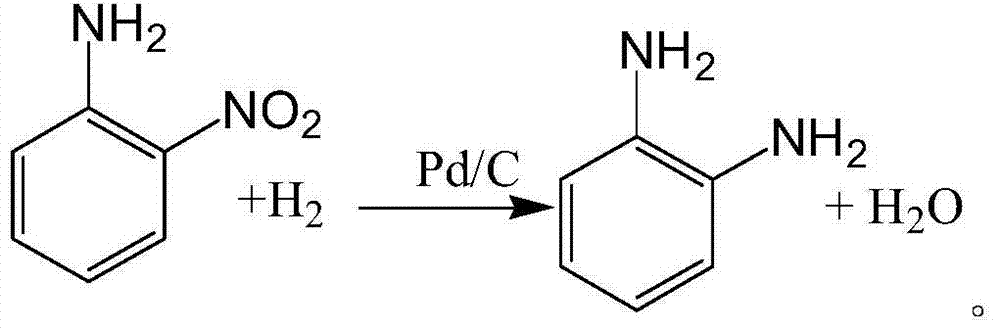
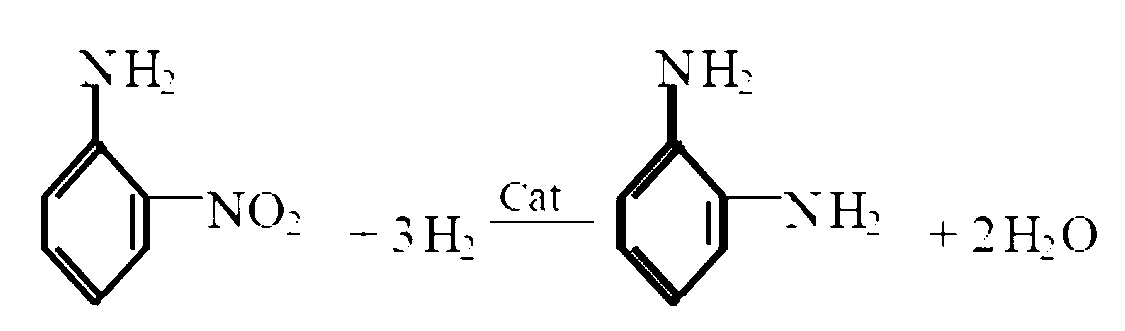
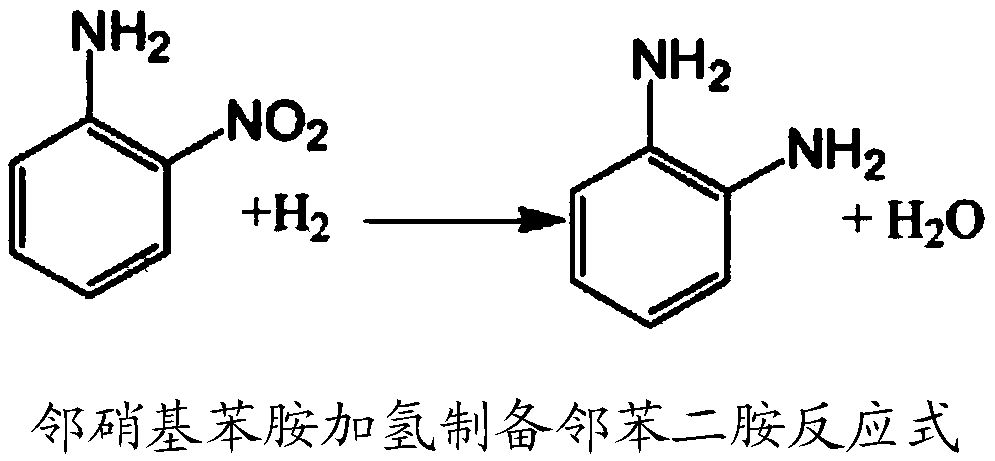
Method for synthesizing o-phenylenediamine from ortho-nitroaniline by virtue of catalytic hydrogenation
InactiveCN104744267APost-processing is simpleGood reaction selectivityOrganic compound preparationChemical recyclingOrtho-nitroanilineFiltration
The invention provides a method for synthesizing o-phenylenediamine from ortho-nitroaniline by virtue of catalytic hydrogenation. The method comprises the following steps: firstly, adding ortho-nitroaniline and a palladium-carbon catalyst into a high-pressure reaction kettle, introducing nitrogen to exhaust air in the kettle, and then introducing hydrogen to exhaust nitrogen in the kettle; secondly, heating and introducing hydrogen, performing catalytic hydrogenation reduction reaction under the conditions that the temperature is 110 DEG C and the pressure is 1.0MPa, reacting for 7-8 hours, then stopping introducing hydrogen, water-cooling, then filtering to recycle the catalyst, and collecting a filtrate after filtration to obtain o-phenylenediamine. According to the method provided by the invention, a raw material namely ortho-nitroaniline is subjected to direct hydrogenation without using a solvent, so that the consumption of the solvent can be greatly reduced, the after-treatment energy consumption can be saved, and large-sized solvent steaming equipment can be saved; and by adopting the method, a separation process can be simplified, the equipment investment, separation energy consumption, material consumption and environmental pollution can be reduced, and the system productivity and production safety can be improved.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
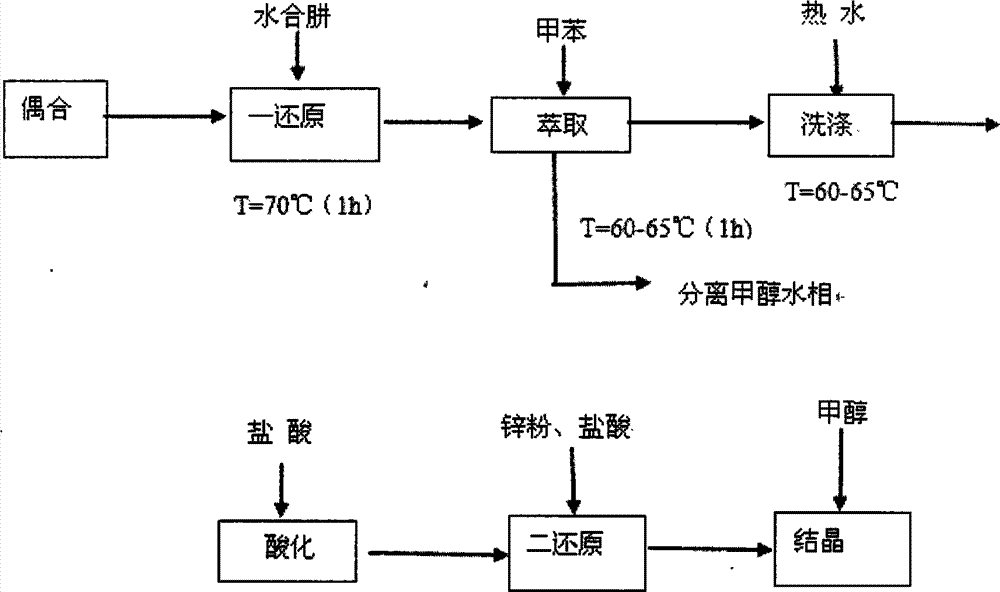
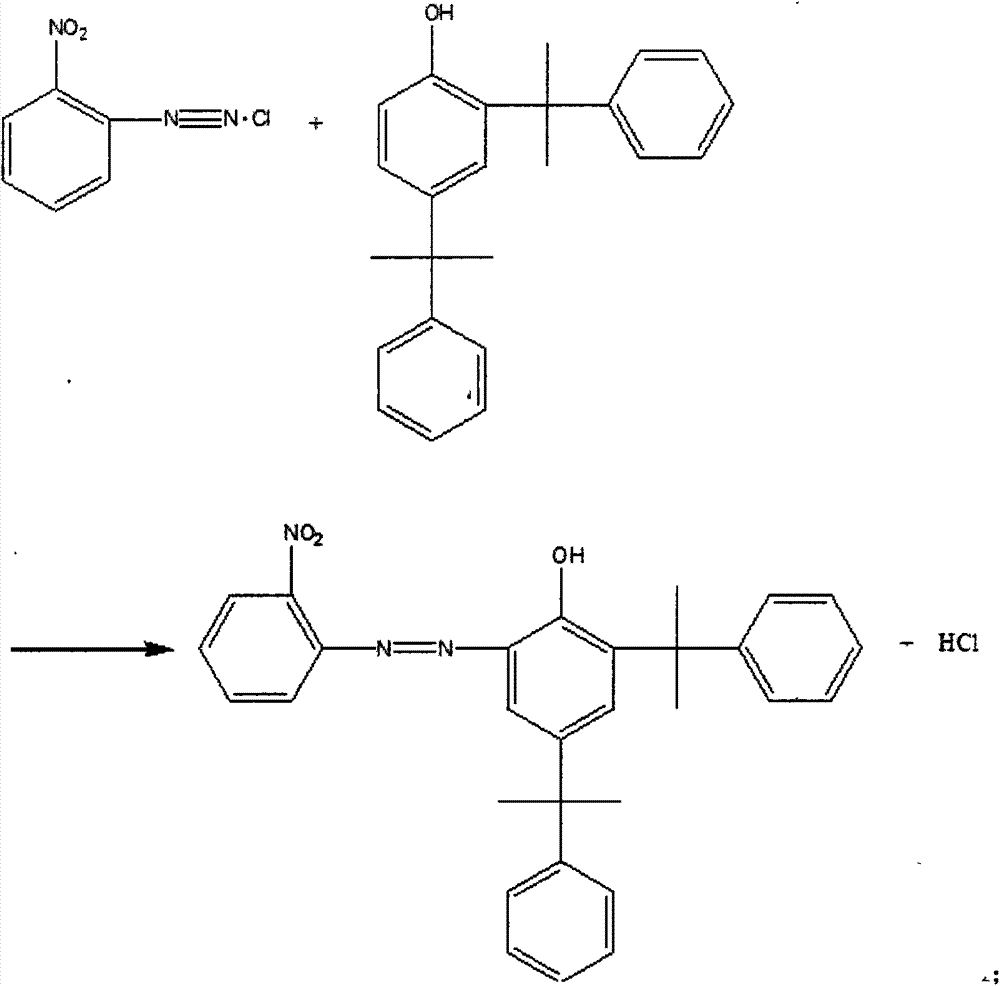
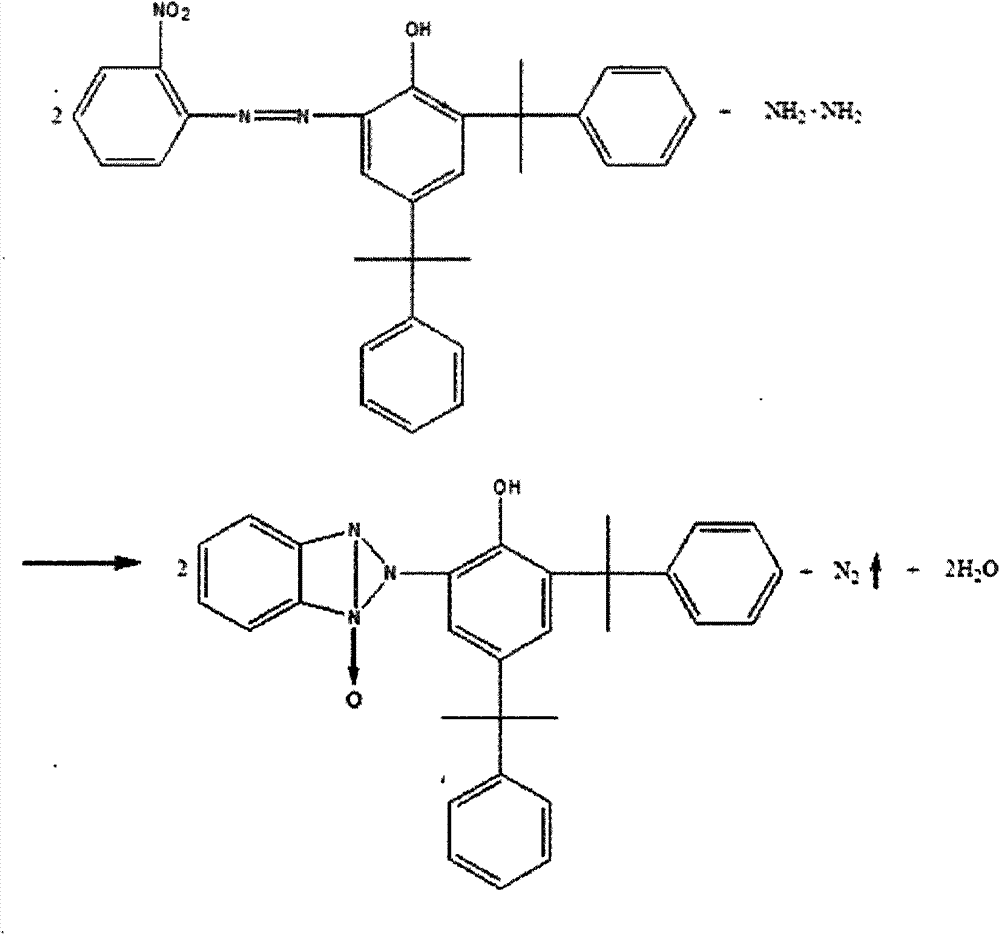
Producing technology of benzotriazole-typed ultraviolet absorber
The invention belongs to the field of a producing technology of an ultraviolet absorber and discloses a producing technology of a benzotriazole-typed ultraviolet absorber. The producing technology includes following steps: mixing ortho-nitroaniline, concentrated hydrochloric acid and water with stirring to obtain a mixed solution; heating the mixed solution to 75 DEG C and cooling the mixed solution to 0 DEG C; adding a solution prepared from sodium nitrite and water to the mixed solution and mixing and stirring the solution with the mixed solution; adding sulfamic acid to obtain a diazonium solution; carrying out a coupling reaction to the diazonium solution with 2,4-dicumenylphenol in an alkaline methanol solution; directly adding hydrazine hydrate after the coupling reaction finished for performing a primary reduction reaction; adding methylbenzene after the primary reduction reaction finished; separating a methanol water phase and recycling methanol through rectification; washing the balanced solvent layer through hot water and heating the solvent layer to 60-65 DEG C; performing an acidizing operation with addition of concentrated hydrochloric acid; adding zinc powder for carrying out a secondary reduction reaction; and adding methanol and performing crystallization to separate out the benzotriazole-typed ultraviolet absorber. The producing technology can effectively shortened technology processes. Purity of an intermediate is improved after extraction. The benzotriazole-typed ultraviolet absorber is reduced in cost.
Owner:DEZHOU HONGKUN MEDICINE INTERMEDIATES CO LTD
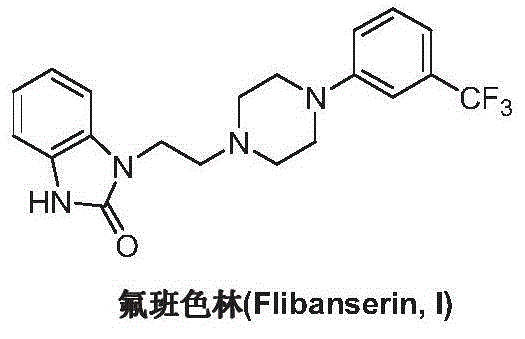
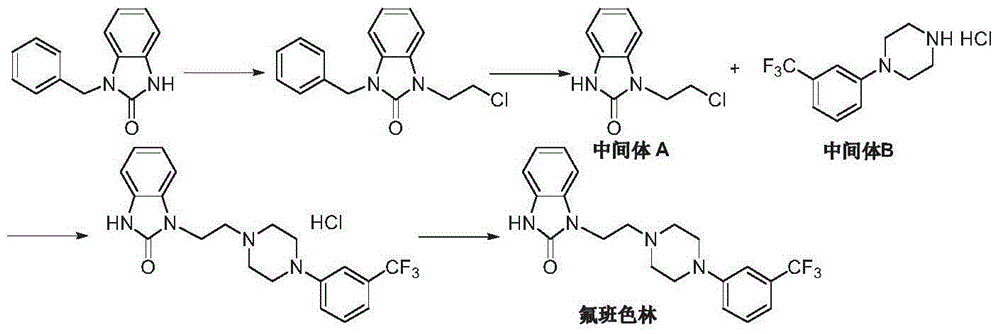
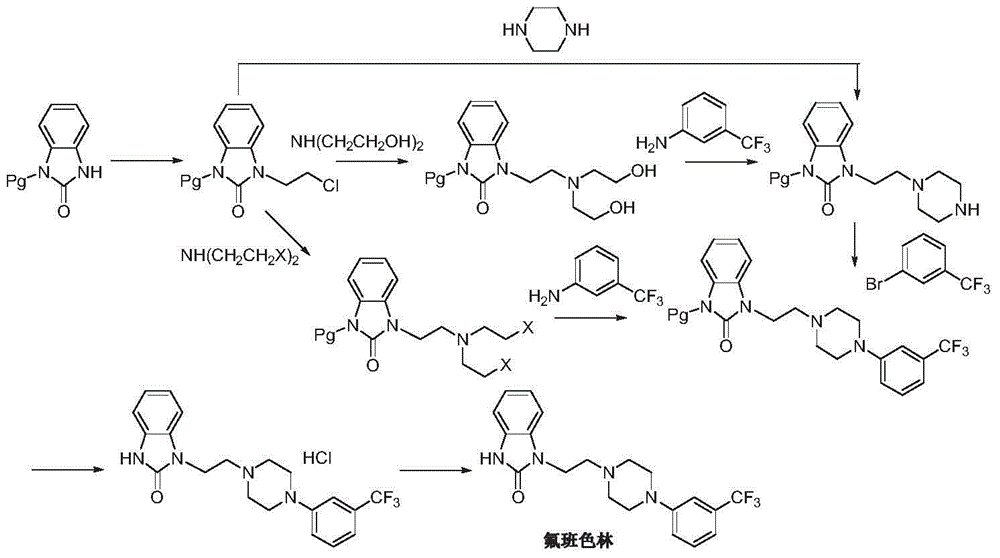
Preparation method of flibanserin
ActiveCN104926734AThe preparation process is convenientHigh yieldOrganic chemistryOrtho-nitroanilineHalogen
The invention discloses a preparation method of flibanserin. The preparation method uses trifluoromethylbenzene, triamine (2-halogen ethyl) and ortho-nitroaniline which are easy to obtain as raw materials and adopts classical elementary reactions such as cyclization, substitution, reduction and condensation, so that the flibanserin is prepared. The raw materials of the preparation method are easy to obtain, the technology is succinct, the yield is high, the preparation method is economical and environment-friendly, and a new preparation way is provided for the industrial production of the flibanserin.
Owner:SUZHOU LIXIN PHARMA
Preparation method of o-phenylenediamine
InactiveCN103012160AReduce manufacturing costAvoid a series of problems such as pollutionOrganic compound preparationAmino compound preparationOrtho-nitroanilineReaction temperature
The invention provides a preparation method of o-phenylenediamine. In a hydrogenation reaction, ortho-nitroaniline is taken as a raw material, and water or organic solvents are not required to be added; under the existence of palladium-platinum bi-metallic catalyst taking activated carbon as a carrier, through series connection of multistage reaction kettles and under the conditions of the reaction temperature of 50 to 150 DEG C and the reaction pressure of 0.5 to 5 MPa, the continuous hydrogenation reduction reaction is carried out; and a reaction liquid flows through a film filtering system, concentrated size containing the catalyst returns to a first-stage reaction kettle, and the filtered clear o-phenylenediamine liquid is dehydrogenized and rectified to obtain the o-phenylenediamine finished product. Compared with the conventional process for preparing o-phenylenediamine through hydrogenation, the method provided by the invention has the advantages that equipment and energy consumption required in subsequent solvent recovery are saved, product quality is good, the production cost is low, the production capacity is high, and the operation is simple and convenient.
Owner:JIANGSU YANGNONG CHEM GROUP +1
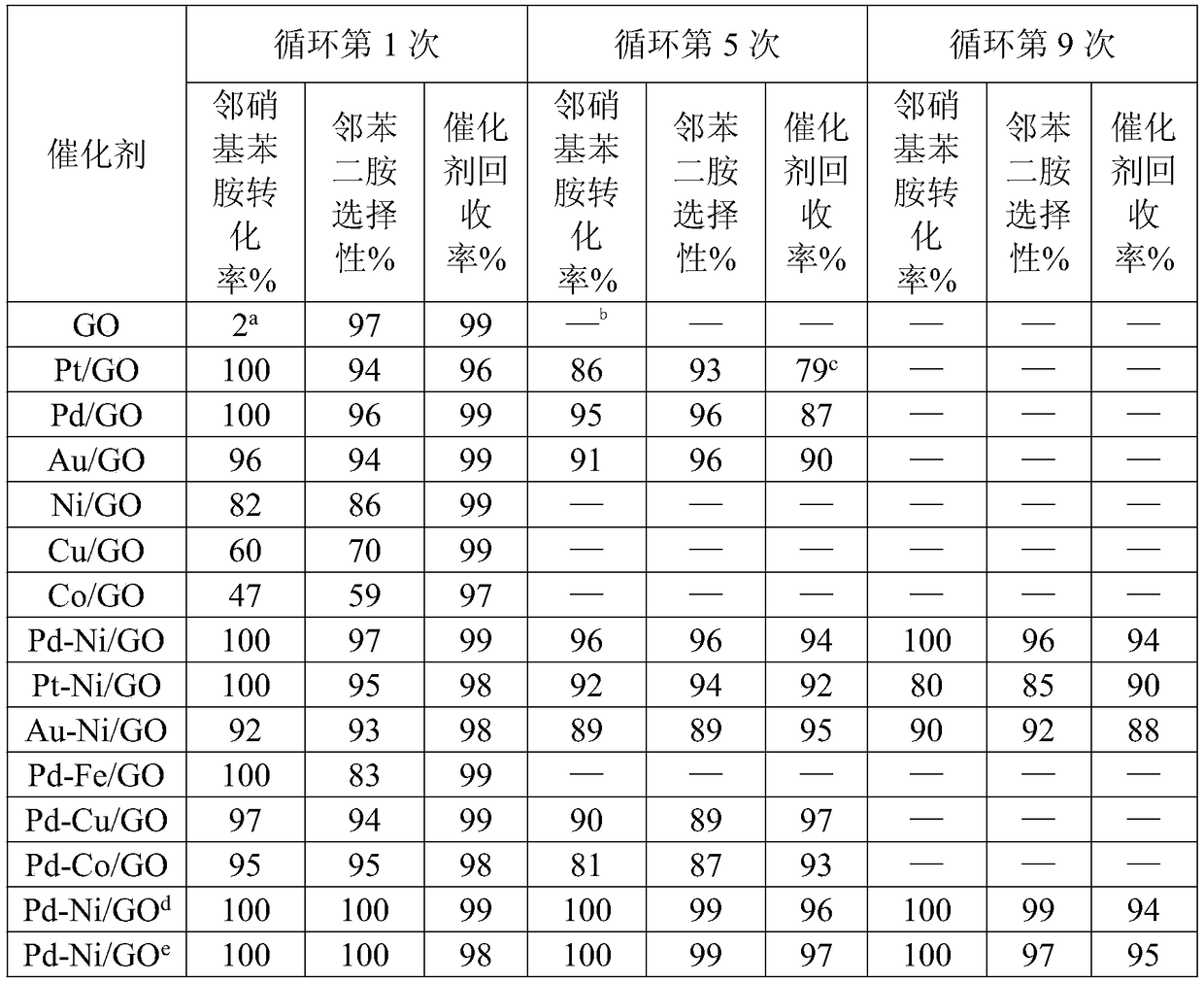
Method for preparing o-phenylenediamine by utilizing catalytic reduction of ortho-nitroaniline
ActiveCN109232271AHigh activityEasy maintenanceOrganic compound preparationAmino compound preparationOrtho-nitroanilineGraphene
The invention provides a method for preparing o-phenylenediamine by utilizing catalytic reduction of ortho-nitroaniline. A bimetal loaded graphene oxide is taken as a catalyst for taking part in hydrogenation reduction in a reaction kettle with a polytetrafluoroethylene liner. According to the scheme, the problems of difficulty in realizing industrial production, difficulty in keeping long-term stable activity of catalyst and the like of the prior art can be solved. The method has a broad industrial application prospect.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Method for preparing gelatin protein and gold nanoparticle composite thin film and application of gelatin protein and gold nanoparticle composite thin film
InactiveCN103084210AHigh reuse rateEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrtho-nitroanilineComposite film
The invention discloses a method for preparing a gelatin protein and gold nanoparticle composite thin film and an application of the gelatin protein and gold nanoparticle composite thin film. By the method for preparing the gelatin protein and gold nanoparticle composite thin film, the gelatin protein and gold nanoparticle composite thin film is prepared by depositing gelatin on a Cu(OH)2 nano wire serving as a filtering layer by a filtering method, performing glue-connection and removing the Cu(OH)2 nano wire and then adsorbing and reducing chloroauric acid radical ions according to a reducing characteristic of the gelatin under the action of charge adsorption of the gelatin. The sizes of gold nanoparticles in the gelatin protein thin film can be controlled by controlling the pH value of a chloroauric acid solution and the reaction time of the gelatin protein thin film in the chloroauric acid solution. The method is simple and feasible; the gelatin protein and gold nanoparticle composite thin film can be used as a catalyst and the catalytic conversion efficiency of ortho-nitroaniline is extremely high; and the gelatin protein and gold nanoparticle composite thin film has the advantages of high repetitiveness and convenience in operation.
Owner:ZHEJIANG UNIV
Method for synthesizing benzimidazole compounds
InactiveCN103288743AImprove conversion rateEasy to separateOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsOrtho-nitroanilineOrganic solvent
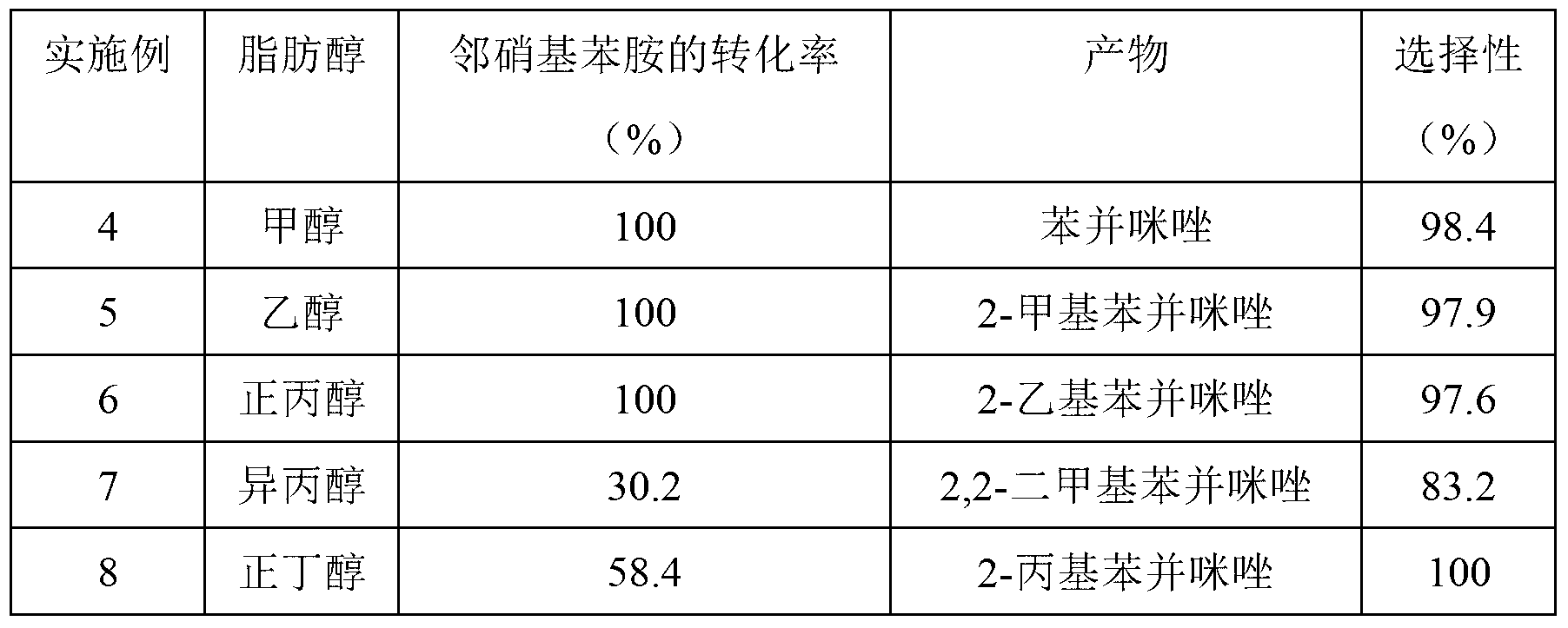
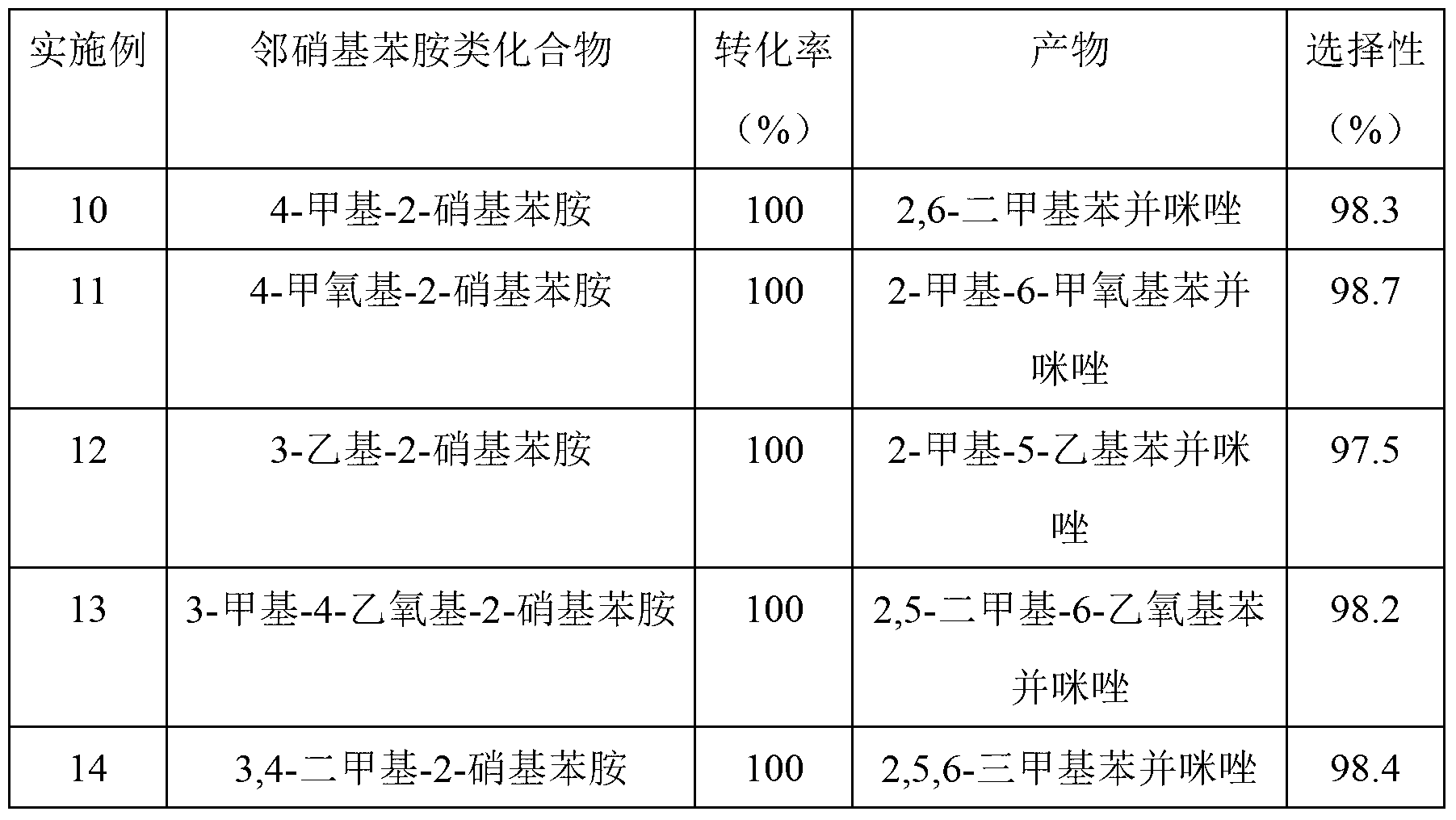
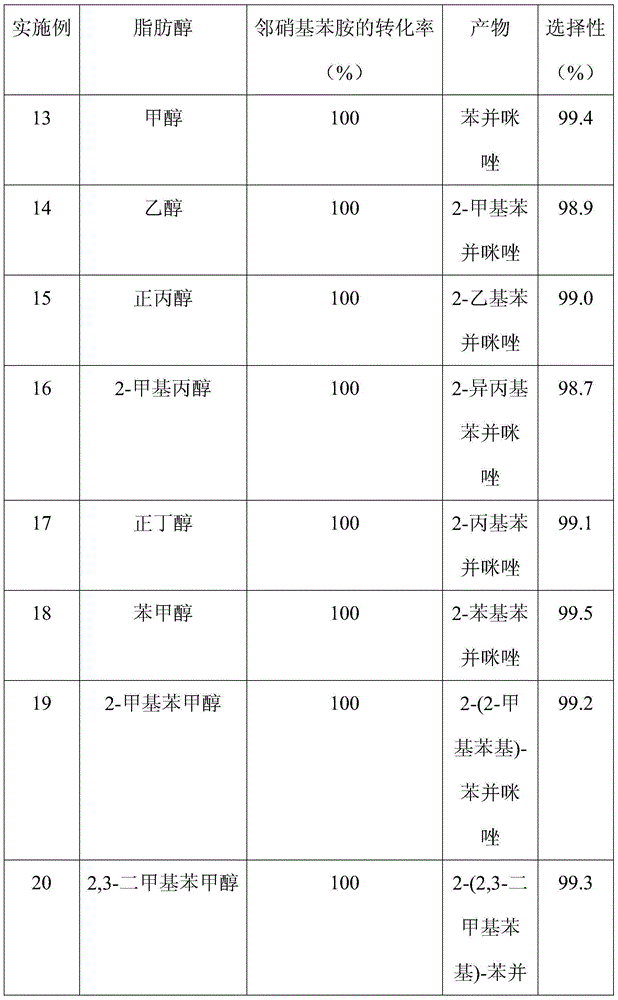
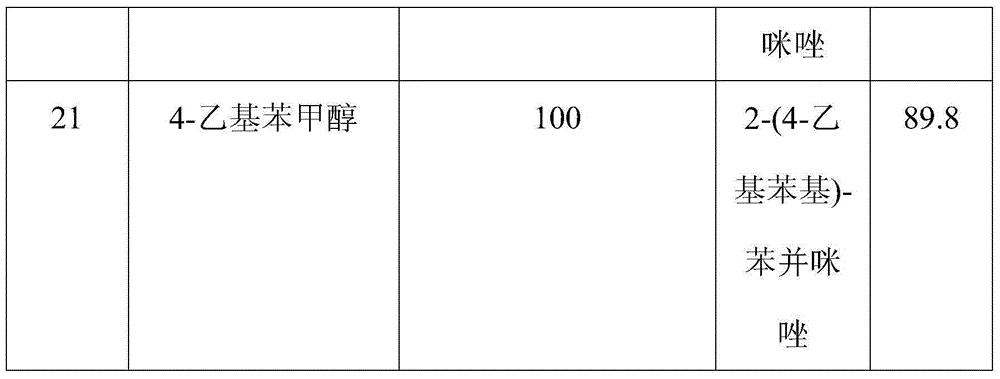
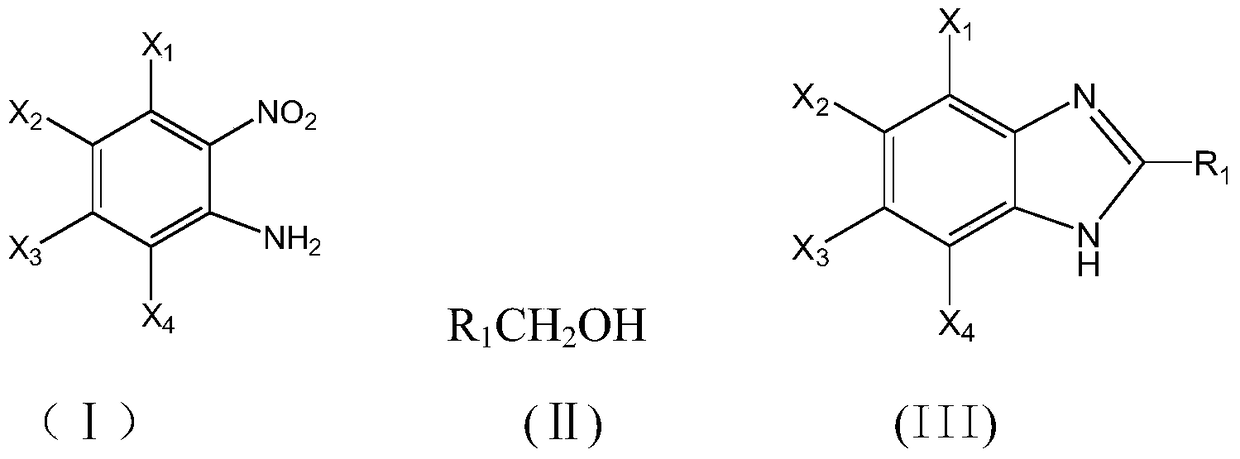
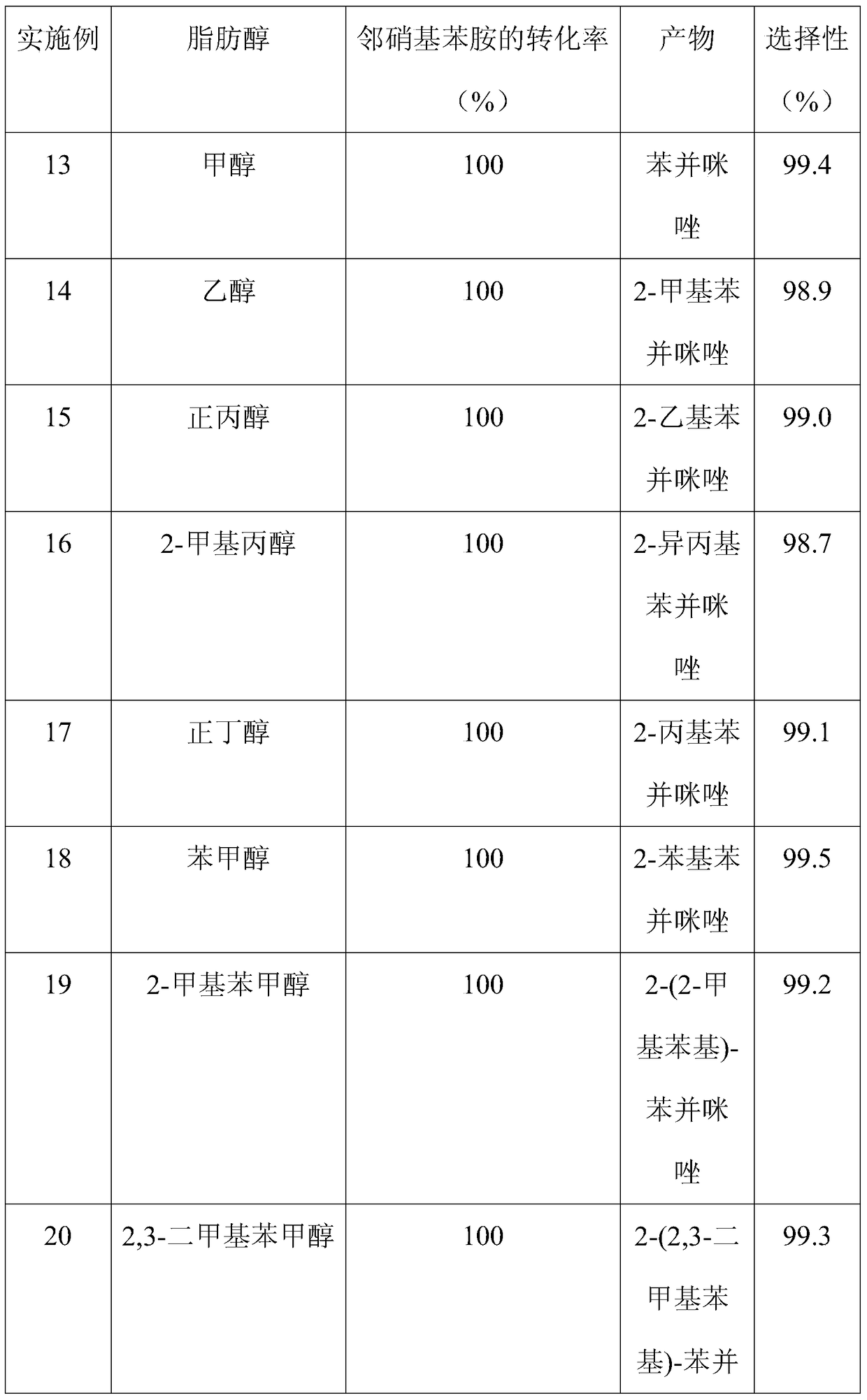
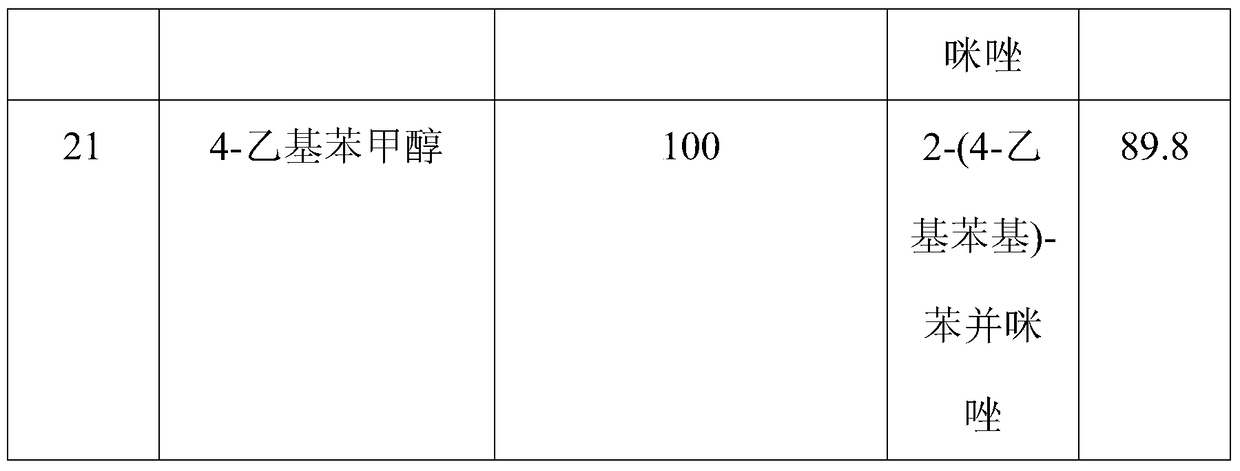
The invention discloses a method for synthesizing benzimidazole compounds expressed by a formula (III) through a one-pot method. The method comprises the following step of: with ortho-nitroaniline compounds expressed by a formula (I) and fatty alcohol expressed by a formula (II) as raw materials as well as water as a reaction solvent, synthesizing the benzimidazole compounds expressed by the formula (III) through the one-pot method in a shielding gas atmosphere under the action of a supported metal solid catalyst, wherein the supported metal solid catalyst is a Cu-Zn-Pd / Al2O3 catalyst. The method disclosed by the invention has the advantages of being simple in synthesis route, high in product yield, low in production cost, liable to separate the selected solid catalyst, high in activity, good in stability and free of liquid acid and organic solvent, and the like, thereby being an environment-friendly synthesis route. FORMUAL (I), R1CH2OH (II), (III) are described in the specification.
Owner:海宁市盐官工业投资有限公司
Method for continuously synthesizing benzimidazole compound
InactiveCN105037274AImprove conversion rateReduce generationOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsLiquid productOrtho-nitroaniline
The invention discloses a method for continuously synthesizing a benzimidazole compound. The method comprises the steps:in a fixed bed reactor, firstly reducing and activating a loaded multi-metal solid catalyst by hydrogen; then using inert gas as carrier gas; adjusting the temperature to 130-250 DEG C and the pressure at 2-10 MPa; continuously introducing mixed raw materials of an ortho-nitroaniline compound shown in a formula (I), fatty alcohol shown in a formula (II) and distilled water iknto the fixed bed reactor by using a high pressure pump; feeding the reactants which are cooled by a condenser to a gas-liquid separator to collect a liquid product so as to obtain the benzimidazole compound shown in a formula (III), wherein the loaded multi-metal solid catalyst is a Cu-Pd-M / Al2O3 catalyst. According to the method disclosed by the invention, multiple intermittent reactions are changed to a one-step continuous reaction, so that the production process is simplified, generation of byproducts is reduced, the conversion rate can reach 100% to the maximum extent, and the yield of the target product benzimidazole compound can reach 99% to the maximum extent. The formulae are shown in the description.
Owner:上海鑫合医药有限公司
Synthesizing method of alpha-carboline compound
InactiveCN107216328AAvoid isomer situationsFacilitate selective reactionOrganic chemistryOrtho-nitroanilineAlpha-carboline
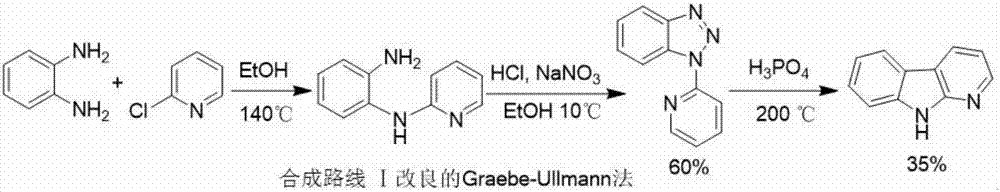
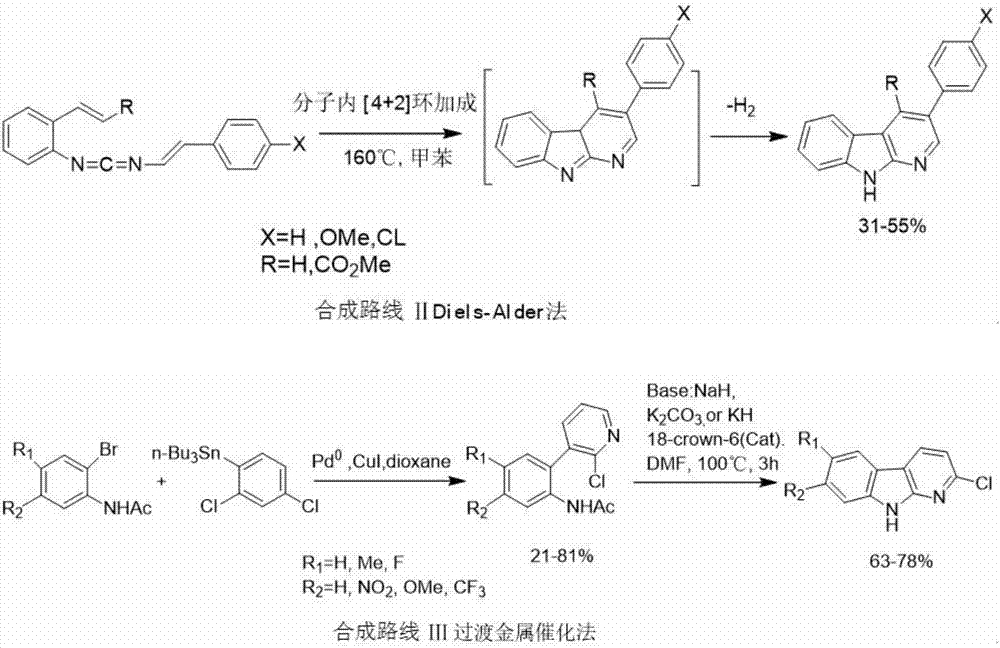
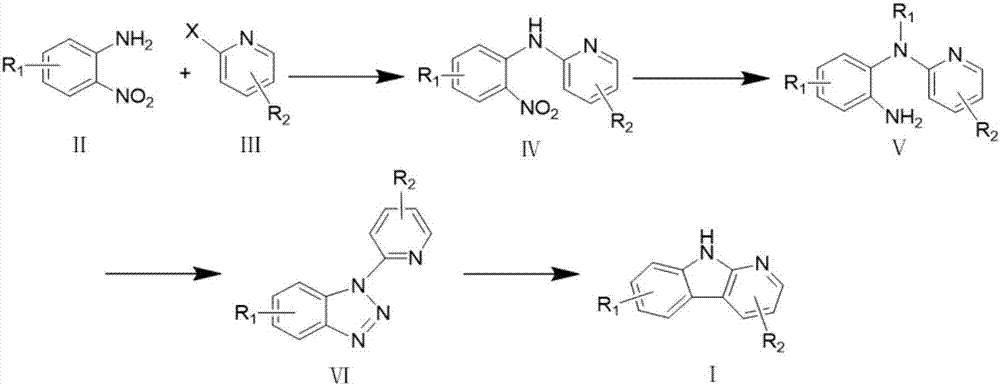
The invention discloses a synthesizing method of an alpha-carboline compound. The synthesizing method comprises the following steps of firstly, enabling ortho-nitroaniline or a derivative thereof to perform typical Ullmann C-N coupling reaction under the action of a catalyst, and performing reduction reaction under the action of a reductant; reacting with nitrous acid under the existence of inorganic acid, and converting into a diazotized triazole intermediate; finally, treating the obtained intermediate after reaction under the action of pyrophosphoric acid or polyphosphoric acid, so as to obtain a target product, namely alpha-carboline or substituted alpha-carboline. The synthesizing method has the characteristics that on the basis of using the improved Graebe-Ullmann method to synthesize the alpha-carboline, the reaction raw materials, reaction reagents and reaction conditions are preferably selected, the selectivity of the target product is enhanced, the reaction time is shortened, the reaction temperature is reduced, the yield rate is increased, the amount of impurities in products is small, the yield rate is high, and the like; the method is conveniently suitable for synthesizing the alpha-carboline and the substituted alpha-carboline, so as to provide a simple and reliable selection solution for the obtaining and utilization of the compound.
Owner:GUIZHOU MEDICAL UNIV
Benzoxazole and benzimidazole compounds and preparation method thereof
The invention discloses a preparation method of benzoxazole and benzimidazole compounds. A specific structural formula of the benzoxazole and benzimidazole compounds is described in the specification, wherein R1 is aryl, substituted aryl or alkyl; R2 is fluorine, chlorine, methoxyl or methyl; and X is oxygen, nitrogen or nitrogen methyl. The preparation method comprises the following steps: taking amino acid and o-nitrophenol (ortho-nitroaniline) compounds sold on the market as starting materials of reaction, and stirring and heating in a solvent under the catalytic action of an alkaline reagent, so that various substituted benzoxazole and benzimidazole compounds can be obtained with high yield. Reaction conditions are mild, no extra oxidizing agent, reducing agent, ligand or transition metal catalyst needs to be added. The preparation method has the advantages of low cost, simple operation and realization of mass production. Experimental results show that the yield of the obtained benzoxazole and benzimidazole compounds can reach 95%.
Owner:XINYANG NORMAL UNIVERSITY
Efficient o-phenylenediamine recovery method
InactiveCN106316863AAvoid bringing inImprove efficiencyAmino compound purification/separationOrganic compound preparationRecovery methodOrtho-nitroaniline
The invention discloses an efficient o-phenylenediamine recovery method. The efficient o-phenylenediamine recovery method mainly comprises the steps that 1, an ortho-nitroaniline and sodium sulfide solution makes ortho-nitroaniline produce reduction reaction; 2, after system cooling, all the materials in the step 1 are pressed into a layering tank for primary layering; 3, an o-phenylenediamine and salt hydrate at the top of the layering tank is transferred to a heating kettle, the materials in the heating kettle are heated to remove moisture in the materials and make the salt precipitate from the o-phenylenediamine, and then collection is performed; 4, the o-phenylenediamine solution obtained after layering is collected; 5, filtration and rectification are performed, and pure o-phenylenediamine is collected and recovered for standby application. By the adoption of the method, the process including cooling, crystallization, centrifugation and heating during rectification is omitted, energy consumption is reduced, the working time is shortened, the phenomenon that a large amount of salt and water is brought to a rectification process is avoided, rectification residues are decreased, and the follow-up o-phenylenediamine rectification quality and yield are improved.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Protein material capable of absorbing ultraviolet ray and preparation method thereof
InactiveCN102408572AEmission reductionImproves UV resistanceVegetal fibresAnimal fibresOrtho-nitroanilineProtein molecules
The invention discloses a protein material capable of absorbing ultraviolet ray and a preparation method thereof. According to the invention, an arylamine compound containing an ortho-nitroaniline structure is subjected to diazotization and then to a coupling reaction with the ortho-position hydroxyl groups of side groups of p-hydroxybenzmethylene in tyrosine residue in protein molecules and produces a structure containing an azo bond with macro-molecular side groups of proteins, and an ortho-hydroxyphenyl-benzotriazole structure is produced through loop closure between the azo bond and ortho-nitro group of an aromatic ring after further treatment with a reducing agent. The ortho-hydroxyphenyl-benzotriazole structure connected with macro-molecular chain of protein has the capability of absorbing ultraviolet ray, which enables a prepared protein material to have the capability of ultraviolet resistance and effectively enhances the functions of ultraviolet resistance and aging resistance of the protein material.
Owner:ZHEJIANG SCI-TECH UNIV
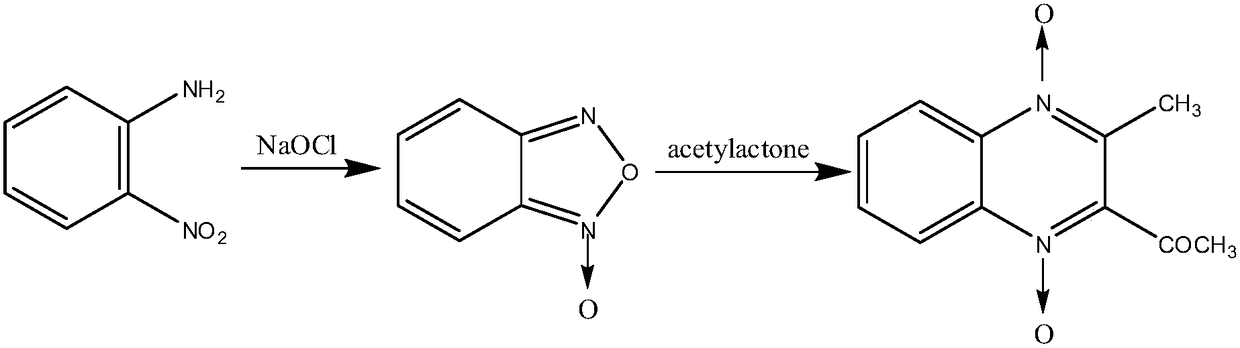
Preparation method of mequindox
ActiveCN109251178AReduce lossesControl activityOrganic chemistryCarboxymethyl celluloseOrtho-nitroaniline
The invention provides a preparation method of mequindox. The preparation method comprises the step of performing reaction by taking ortho-nitroaniline, sodium hypochlorite and acetylacetone as raw materials, taking an NaOH / attapulgite compound as a catalyst and taking sodium carboxymethyl cellulose as a solubilizing agent and a homogenizing agent so as to obtain the mequindox. The preparation method of the mequindox, provided by the invention, realizes the purpose of one-step preparation of the mequindox, omits the intermediate treatment step of benzofurazan, and is simple in technology and high in production efficiency. The purity of the prepared mequindox product can reach 99% or more, and the total yield of the prepared mequindox product can reach 84.5% or more, which is equivalent tosingle-step yield of 90% or more, so that the prepared mequindox product has broad application prospects.
Owner:河北美荷药业有限公司
Agent for simultaneously bleaching and coloring of keratin fibres comprising an anionic or non-ionic dye and an inert organic liquid
InactiveCN1899244AImprove stabilityEasy to useCosmetic preparationsHair cosmeticsFiberOrtho-nitroaniline
The present disclosure relates to a composition for simultaneously bleaching and dyeing keratin fibers, comprising at least one direct dye chosen from anionic dyes, nonionic dyes, and addition salts thereof, with the exception of 7-(6'-methylphenylazo)-1-acetamido-3,6-disulfo-8-hydroxynaphthalene, ortho-nitroanilines substituted meta to the amino group, quinoline and quinoline derivatives, and addition salts thereof, at least one inert organic liquid, at least one peroxygenated salt and at least one alkaline agent. The disclosure also relates to process and use for simultaneously bleaching and dyeing keratin fibers using this composition. Especially suitably used for dark hair, capable of being easily used, and capable of rapidly bringing about coloration with a chromatic color.
Owner:LOREAL SA
O-phenylenediamine preparation process by means of hydrogenization with nano nickel serving as catalyst
InactiveCN102633654ASolve the problem of a large amount of organic wastewater produced by reductionReduce corrosionOrganic compound preparationAmino compound preparationChemical industryOrtho-nitroaniline
The invention discloses an o-phenylenediamine preparation process by means of hydrogenization with nano nickel serving as a catalyst. The o-phenylenediamine is prepared by means of reduction process with ortho-nitroaniline serving as a raw material and alcohol serving as solvent. The process is characterized in that nano nickel and hydrogen which serve as catalysts are added during reaction, the hydrogen pressure ranges from 0.5MPa to 1.5MPa, the reaction temperature is below 30-80 DEG C, and the o-phenylenediamine is obtained by rectification after reduction for 0.5-10h. By means of the nano nickel catalyst with the patent application number being 201110059900.X and made by Jiangsu Kangheng chemical industry Co.,Ltd, the problem of a great quantity of organic waste water generated in reduction of iron powder or sodium sulfide in the traditional art is solved. The hydrogenization process avoids concentrated acid and concentrated base which are used in the traditional art, so that equipment corrosion is greatly reduced, pollution is reduced, and approximate zero pollution is achieved. Further, product yield and quality are improved, equipment production efficiency is improved, and energy consumption is greatly reduced.
Owner:JIANGSU KANGHENG CHEM
O-phenylenediamine medium-pressure catalytic hydrogenation process
InactiveCN103787893AGood applicationLow reaction temperatureOrganic compound preparationAmino compound preparationOrtho-nitroanilineReaction temperature
The invention discloses an o-phenylenediamine medium-pressure catalytic hydrogenation process which is characterized by comprising the following steps: adding ortho-nitroaniline as a raw material, water as a solvent and 5% of palladium-carbon as a catalyst into a pressure kettle, repeatedly replacing the air inside the kettle through nitrogen, subsequently emptying the kettle, further repeating the replacement by using hydrogen, after the replacement is accomplished, raising the temperature, heating, reacting for 2 hours, after the reaction is accomplished, keeping the pressure, reacting for 0.5 hour, after the reaction is accomplished, cooling down, replacing the hydrogen inside the kettle by using nitrogen, discharging the material in hot, filtering the catalyst for further application, cooling down the filtrate to separate out crystal, cooling down and filtering to obtain a product, namely, the o-phenylenediamine, refeeding the filtrate into a next batch for being used as process water for repeated circulation. The further application situation of the catalyst is good, the reaction temperature is low, the pressure is small, the synthesis cost is greatly lowered, and in addition the process water is repeatedly applied, and the wastewater is discharged for only once after the process water is applied for multiple times, so that the water pollution is greatly reduced.
Owner:NANTONG BOTAO CHEM
Synthesis method for 3-amino-1,2,4-phentriazine-1,4-dioxide
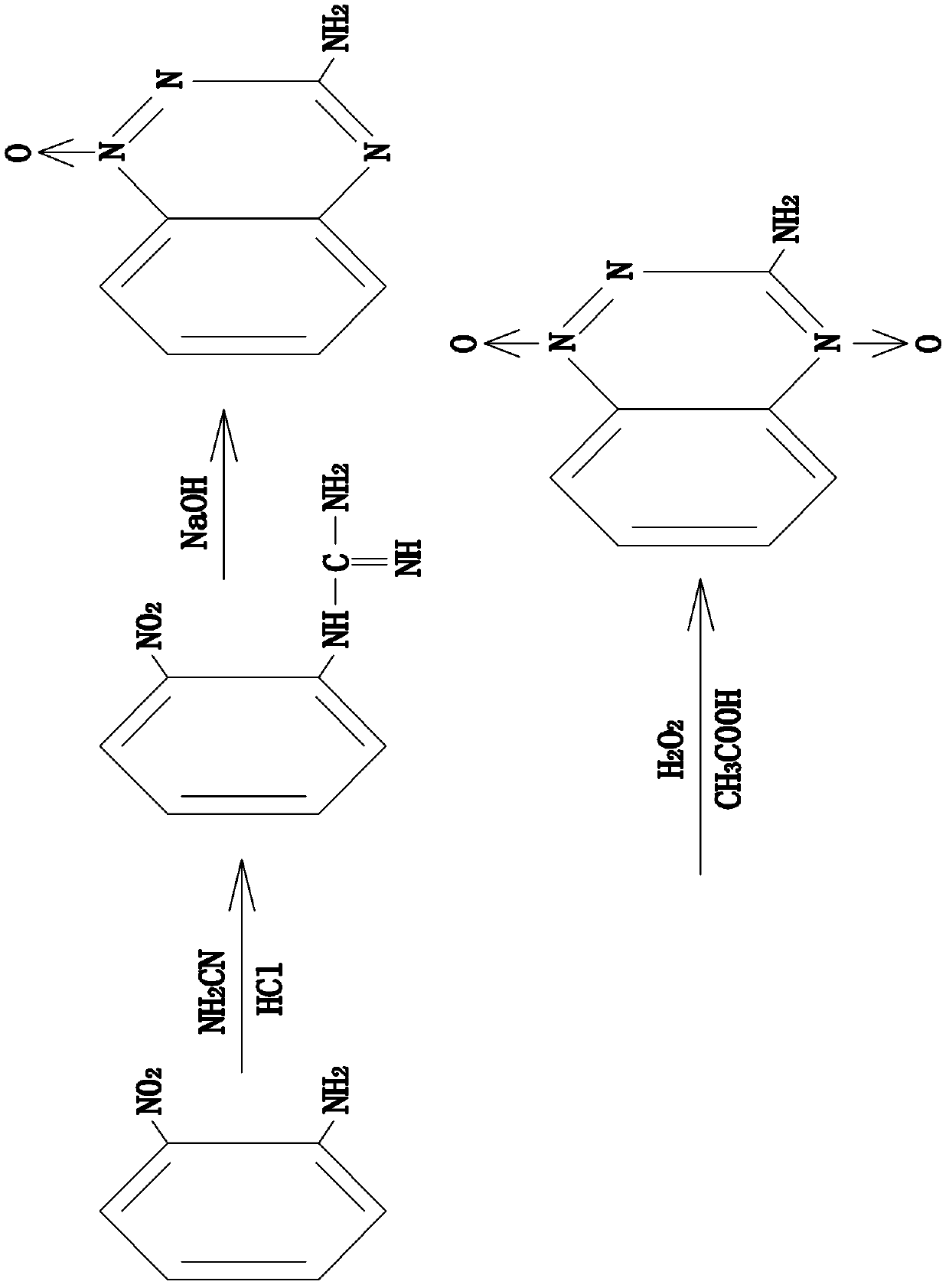
InactiveCN103373968AAdequate responseStable harvestOrganic chemistryOrtho-nitroanilineSynthesis methods
The invention relates to a synthesis method for 3-amino-1,2,4-phentriazine-1,4-dioxide. The invention provides a synthesis method for 3-amino-1,2,4-phentriazine-1,4-dioxide, which is low in toxicity, moderate in reaction conditions, simple to operate, high in safety, and stable in yield. The method disclosed by the invention comprises the following steps of: firstly, co-heating ortho-nitroaniline in 55% cyanamide aqueous solution and concentrated HCl, cooling, then adding NaOH solution, and finishing a ring-closure reaction to generate an intermediate product, namely, 3-amino-1,2,4-phentriazine-1-oxide; secondly, adding the intermediate product, namely, 3-amino-1,2,4-phentriazine-1-oxide in CH3COOH to form a suspended matter, then adding H2O2 solution, and reacting in the dark to obtain 3-amino-1,2,4-phentriazine-1,4-dioxide solution; and finally, performing rotary evaporation on the obtained 3-amino-1,2,4-phentriazine-1,4-dioxide solution to remove a solvent, separating out a red solid substance, filtering, and recrystallizing a filter cake by absolute ethyl alcohol to obtain a 3-amino-1,2,4-phentriazine-1,4-dioxide crystal.
Owner:由国峰
Continuous ammoniation method of aniline organic intermediates
InactiveCN107382747ALower synthesis costHigh yieldOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneOrtho-nitroaniline
The invention discloses a continuous ammoniation method of aniline organic intermediates. The continuous ammoniation method concretely comprises the following steps of feeding o-nitrochlorobenzene and concentrated ammonia liquid into a six-stage serially connected high-pressure autoclave in a continuous feeding and continuous discharging mode; performing reaction for 10 to 15 hours at the inside pressure of 5.3MPa and the reaction temperature being 170 DEG C; obtaining ortho-nitroaniline; then, transferring the reaction liquid into a secondary reaction kettle; adding caustic alkali for regulating the pH value to be 12 to 13; then, performing reaction for 10 to 30min; obtaining high-purity ortho-nitroaniline and byproducts of ammonium chloride. The continuous ammoniation method has advantages that useful substances can be effectively recovered from intermediates of ortho-nitroaniline produced from carbendazim after the primary reaction is completed; the content of the ortho-nitroaniline after the hydrogenation is 98 percent or higher; the synthesis cost is reduced; the economic benefits are improved; the ammoniation yield is improved; the continuous ammoniation method and process design of the aniline organic intermediates are optimized; the continuous ammoniation method and process design requirements of the aniline organic intermediates are met.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
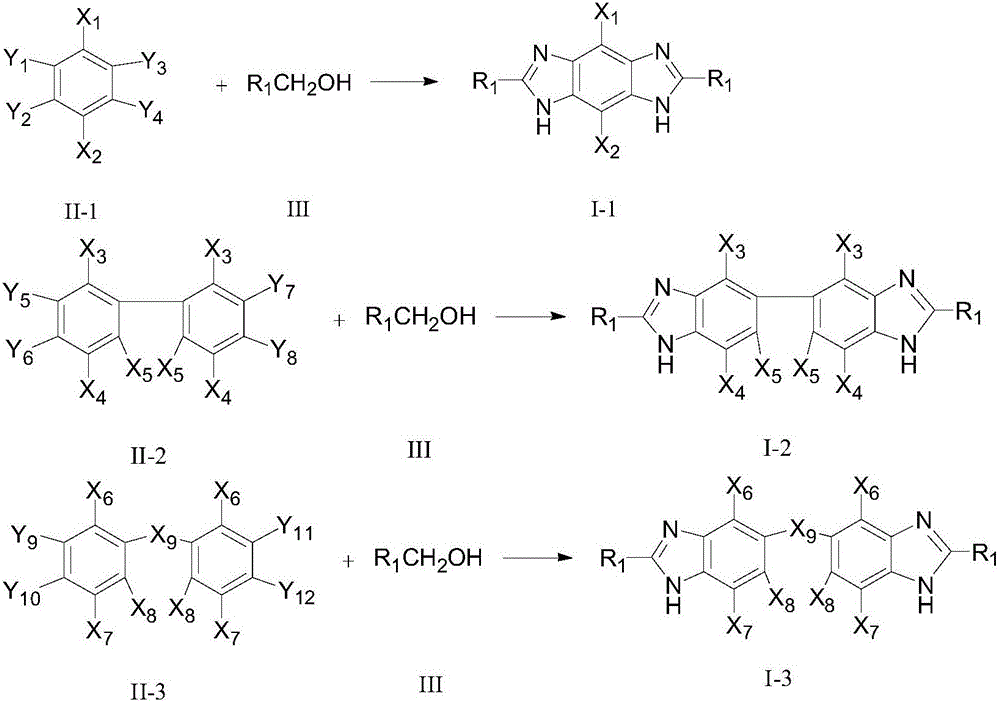
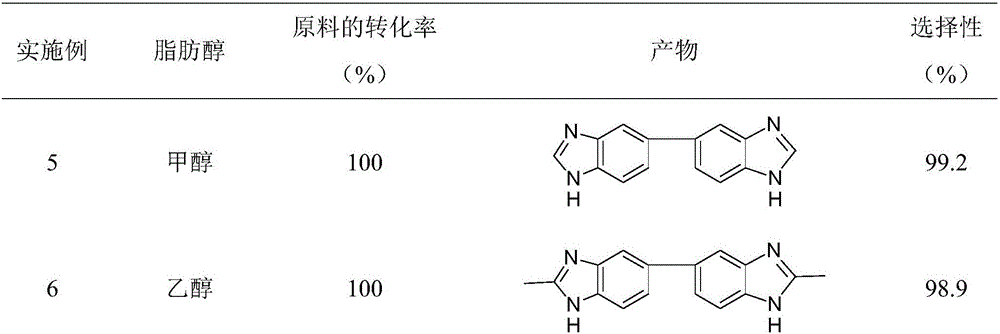
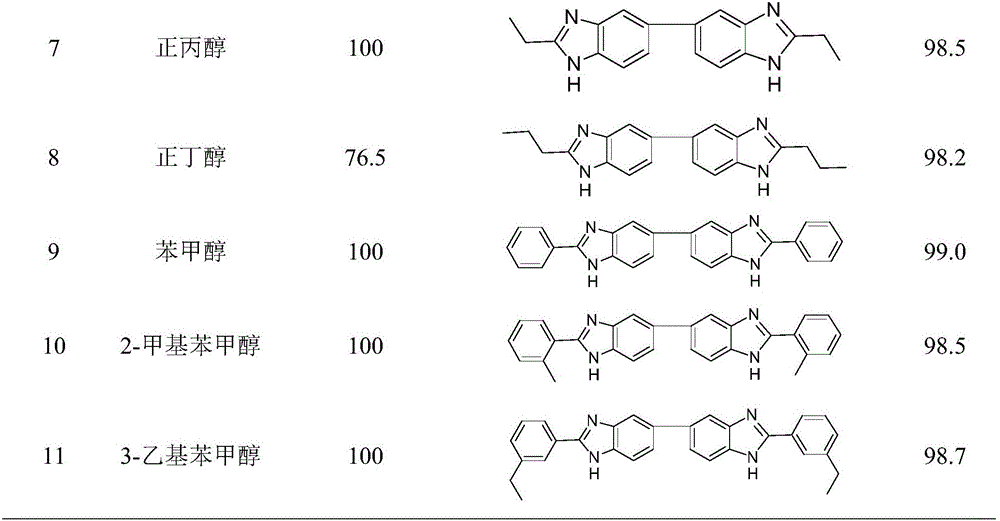
Method for synthesizing bis-benzimidazole compound through one-pot method
ActiveCN105777650AEasy to separateReduce generationOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsOrtho-nitroanilineAlcohol
The invention provides a method for synthesizing a bis-benzimidazole compound through a one-pot method.According to the method, a bis(ortho-nitroaniline) compound shown in a formula II-1 or II-2 or II-3 and alcohol shown in a formula III are used as raw materials, water and organic solvent are added, and under the effect of a load type multi-metal solid catalyst, the bis-benzimidazole compound shown in the corresponding formula II-1 or II-2 or II-3 is synthesized through the one-pot method, wherein X1, X2, X3, X4, X5, X6, X7 and X8 are H, F, C1, CH3, CH2CH3 and OCH3 or OCH2CH3 independently; X9 is O or CO or CH2 or NH; R1 is H or alkyl or phenyl or alkyl phenyl or alkoxy phenyl, and the number of carbon atoms of alkyl and alkoxy is 1-3; in Y1 and Y2, Y3 and Y4, Y5 and Y6, Y7 and Y8, Y9 and Y10, and Y11 and Y12, one in each group is amino, and the other in each group is nitryl.The method has the advantages that the synthetic route technology is simple, the product yield is high, the production cost is low, the catalyst is easy to separate, high in activity and good in stability, and liquid acid is not used.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing benzofuroxan under catalysis of surfactant micelle
InactiveCN104945347AEasy to purifyReduce pollutionOrganic chemistryOrtho-nitroanilineSodium chlorate
The invention provides a method for preparing benzofuroxan under catalysis of surfactant micelle. According to the method, water is used as a solvent; oxidization of ortho-nitroaniline and derivatives of ortho-nitroaniline with NaClO to prepare benzofuroxan is catalyzed by micelle made of a surfactant. The method comprises the following steps: adding a certain quantity of water, a certain quantity of alkali, a small quantity of the surfactant and a small quantity of ortho-nitroaniline into a reactor; dripping a sodium hypochlorite solution at room temperature; carrying out reaction for two hours after dripping is finished; carrying out filtration to obtain the benzofuroxan product. The method has the advantages that the conditions are mild; the process is simple; the purity of the obtained product is relatively high; purification and crystallization are easy; the quantity of organic contents in the effluent after reaction is low; environmental pollution is low; the environment is protected; suitability for industrial large-scale production is high.
Owner:中国兵器工业第二一三研究所
Preparation method of ultraviolet light absorber UV-PS
InactiveCN105884703AAvoid reducing yieldCarbon chain lengthOrganic chemistryOrtho-nitroanilineUltraviolet lights
The invention discloses a preparation method of ultraviolet light absorber UV-PS as indicated in the formula (I). The preparation method comprises the steps of 1, mixing ortho-nitroaniline with hydrochloric acid, then adding sodium nitrite, and conducting a reaction for 1-3 hours with the temperature preserved; adding urea for a reaction, so that a diazonium salt solution is obtained; 2, mixing and dissolving first solvent, an auxiliary agent and tert-butylphenol, lowering the temperature, adding the diazonium salt solution, adding alkali, and preserving the temperature for 1-3 hours; 3, conducting purification for the first time on a mixed solution obtained in step 2, so that azo matter as indicated in the formula (II) is obtained; 4, mixing the azo matter, alkali and second solvent for a reaction, raising the temperature to 60-70 DEG C, adding glucose, conducting backflow for 2-4 hours, and adding acid; 5, conducting purification for the second time on a mixed solution obtained in step 4, so that an intermediate as indicated in the formula (III) is obtained; 6, mixing the intermediate, third solvent and an auxiliary agent for a reaction, raising the temperature to 80-85 DEG C, adding zinc powder, conducting a reaction for 2.5-3 hours, and adding adsorbent; 7, conducting purification for the third time on a mixed solution obtained in step 6, so that the ultraviolet light absorber UV-PS as indicated in the formula (I) is obtained. According to the preparation method of the ultraviolet light absorber UV-PS, a fractional-step method is adopted, reactant in each step can fully react, the yield of products of all steps is guaranteed, and the yield of a final product is remarkably raised.
Owner:启东金美化学有限公司
Method for synthesizing azophenylene amide compound with antineoplastic activity and application of azophenylene amide compound
ActiveCN105418519AToxicClinical drug safetyOrganic chemistryAntineoplastic agentsOrtho-nitroanilineChloroacetyl chloride
The invention relates to a method for synthesizing an azophenylene amide compound with antineoplastic activity and an application of the azophenylene amide compound. The method includes the following steps that aniline hydrochloride and ortho-nitroaniline are reacted under the catalyst condition, processing is carried out after the reaction is finished, and a red solid azophenylene-2-amine is obtained; the azophenylene-2-amine is dissolved into dichloromethane, then triethylamine is added into the reaction liquid, the reaction liquid is stirred to be even and cooled to the temperature lower than 15 DEG C, a chloroacetyl-chloride-dissolving dichloromethane solution is slowly added into the reaction liquid, the mixture is stirred and reacted for 30-60 min under the condition of -15 DEG C, solvents are removed, a crude product is obtained, and a light-yellow solid 2-chlorine-N-(2-azophenylene)acetamide is obtained after the crude product is purified. The method has the advantages that according to the azophenylene amide compound with the antineoplastic activity, clinical medication is safe, the preparing method is simple, raw material sources are convenient, industrialization can be achieved, the requirements of antineoplastic medicine can be met, and the azophenylene amide compound is expected to be developed into new antineoplastic medicine.
Owner:高小春
Method for preparing ortho-nitroaniline by high pressure ammonolysis
InactiveCN103864625ASolve the problem of difficult wastewater treatmentMeet the requirements of green environmental protectionOrganic compound preparationAmino compound preparationOrtho-nitroanilineWastewater
The invention discloses a method for preparing ortho-nitroaniline by high pressure ammonolysis. The method comprises the following steps: enabling o-chloronitrobenzene and liquid ammonia to generate an ammonolysis reaction under the high pressure to generate a reaction mixture containing o-chloronitrobenzene, unreacted liquid ammonia and ammonium chloride which is a byproduct, recycling the unreacted liquid ammonia from the reaction mixture, then adding a solvent to the rest reaction mixture, removing ammonium chloride solid through filtering, and removing the solvent from filtrate to obtain the ortho-nitroaniline, wherein the high pressure (gauge pressure) is 6-10MPa. According to the method disclosed by the invention, the problem that a great deal of wastewater containing ammonium chloride, which is difficult to treat, is generated in the traditional process is solved. Therefore, the method disclosed by the invention is low in production cost, simple to operate, free of generation of wastewater and environment-friendly and has a favorable industrial application prospect.
Owner:凯美泰克(天津)化工科技有限公司
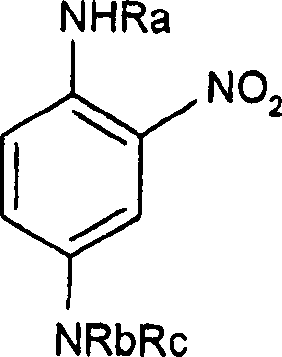
Composition for bleaching and simultaneously dyeing keratin fibers, comprising meta-substituted ortho-nitroaniline
The present invention relates to a composition for bleaching and simultaneously dyeing keratin fibers, comprising at least one dye chosen from meta-substituted ortho-nitroanilines and addition salts thereof, at least one peroxygenated salt and at least one alkali agent, to a method for bleaching and dyeing keratin fibers using the composition, and also to the use of the composition for bleaching and simultaneously dyeing keratin fibers. The composition in accordance with the present invention is particularly suitable for dark hair. It has improved stability over time and allows chromatic and fast dyeing to be obtained.
Owner:LOREAL SA
A kind of method for continuous synthesis of benzimidazole compounds
InactiveCN105037274BReduce generationSimple production processOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsLiquid productOrtho-nitroaniline
The invention discloses a method for continuously synthesizing a benzimidazole compound. The method comprises the steps:in a fixed bed reactor, firstly reducing and activating a loaded multi-metal solid catalyst by hydrogen; then using inert gas as carrier gas; adjusting the temperature to 130-250 DEG C and the pressure at 2-10 MPa; continuously introducing mixed raw materials of an ortho-nitroaniline compound shown in a formula (I), fatty alcohol shown in a formula (II) and distilled water iknto the fixed bed reactor by using a high pressure pump; feeding the reactants which are cooled by a condenser to a gas-liquid separator to collect a liquid product so as to obtain the benzimidazole compound shown in a formula (III), wherein the loaded multi-metal solid catalyst is a Cu-Pd-M / Al2O3 catalyst. According to the method disclosed by the invention, multiple intermittent reactions are changed to a one-step continuous reaction, so that the production process is simplified, generation of byproducts is reduced, the conversion rate can reach 100% to the maximum extent, and the yield of the target product benzimidazole compound can reach 99% to the maximum extent. The formulae are shown in the description.
Owner:上海鑫合医药有限公司
Protein material capable of absorbing ultraviolet ray and preparation method thereof
InactiveCN102408572BEmission reductionImproves UV resistanceOrganic chemistryVegetal fibresOrtho-nitroanilineProtein molecules
The invention discloses a protein material capable of absorbing ultraviolet ray and a preparation method thereof. According to the invention, an arylamine compound containing an ortho-nitroaniline structure is subjected to diazotization and then to a coupling reaction with the ortho-position hydroxyl groups of side groups of p-hydroxybenzmethylene in tyrosine residue in protein molecules and produces a structure containing an azo bond with macro-molecular side groups of proteins, and an ortho-hydroxyphenyl-benzotriazole structure is produced through loop closure between the azo bond and ortho-nitro group of an aromatic ring after further treatment with a reducing agent. The ortho-hydroxyphenyl-benzotriazole structure connected with macro-molecular chain of protein has the capability of absorbing ultraviolet ray, which enables a prepared protein material to have the capability of ultraviolet resistance and effectively enhances the functions of ultraviolet resistance and aging resistance of the protein material.
Owner:ZHEJIANG SCI-TECH UNIV
Synthetic method of 8-nitroquinoline
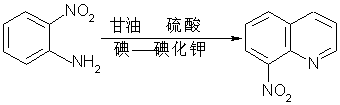
InactiveCN109160900AModerate and controllable dehydration reactionModerate and controllable cyclization reactionOrganic chemistryOrtho-nitroanilineGas phase
The invention provides a synthetic method of 8-nitroquinoline. The synthetic method comprises the following steps: taking ortho-nitroaniline and glycerinum as raw materials, carrying out dehydration in sulfuric acid, and taking iodine-potassium iodide as an oxidizing agent to cyclize 8-nitroquinoline. At the temperature of 60 to 120 DEG C, glycerinum is added dropwise, the dehydration reaction ofglycerinum and sulfuric acid is mild and controllable, and then the cyclization reaction with ortho-nitroaniline is mild and controllable quickly, so that heat release controllability of sharp reaction cannot be caused. An iodine-potassium iodide water solution is used for taking the place of diarsenic pentoxide as an oxidant, the synthetic method is safe and pollution-free, simple in operation and relatively high in yield. The iodine-potassium iodide catalyst is mild, when a back flow becomes clear from muddy, the reaction is basically finished, the phenomenon is obvious, the reaction endpoint is easy to control, gas phase tracking detection can be realized, the post-treatment is simple, little waste water is generated, and the production process is safe and environmentally-friendly.
Owner:HUAIAN WAN BANG SPICE IND CO LTD +1
Corrosion-resistant SBS modified asphalt formula
InactiveCN109439007AImprove performanceImprove tensile propertiesBuilding insulationsOrtho-nitroanilinePolyvinyl alcohol
The invention relates to a corrosion-resistant SBS modified asphalt formula which consists of the following raw materials: 75-90 parts of asphalt, 18-29 parts of SBS, 20-25 parts of butadiene styrenerubber, 6-10 parts of polyvinyl alcohol, 1-5 parts of a sodium hydroxide powder, 0.5-1.5 parts of vulcanized rubber, 3-10 parts of hexachloro-p-xylene, 4-9.5 parts of synthetic fatty acid, 4-9.5 partsof synthetic sodium aliphatate, 1-2.5 parts of borate mineral, 3-7 parts of castor oil, 0.5-3 parts of coal tar, 4-9.5 parts of phenyl salicylate, 1-3.5 parts of ortho-nitroaniline, 0.85-4 parts of asand powder, 2-5 parts of a polysulfide sealant, 1-3 parts of polycarbonate and 1-3 parts of polyacrylonitrile.
Owner:许谐兴
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com