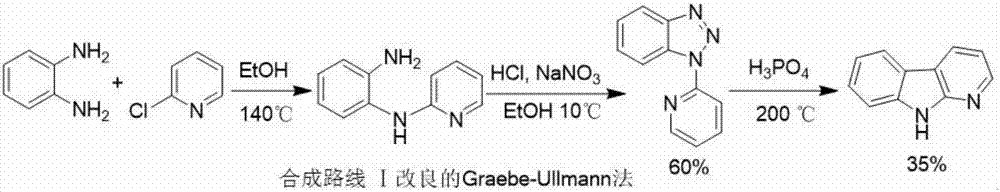
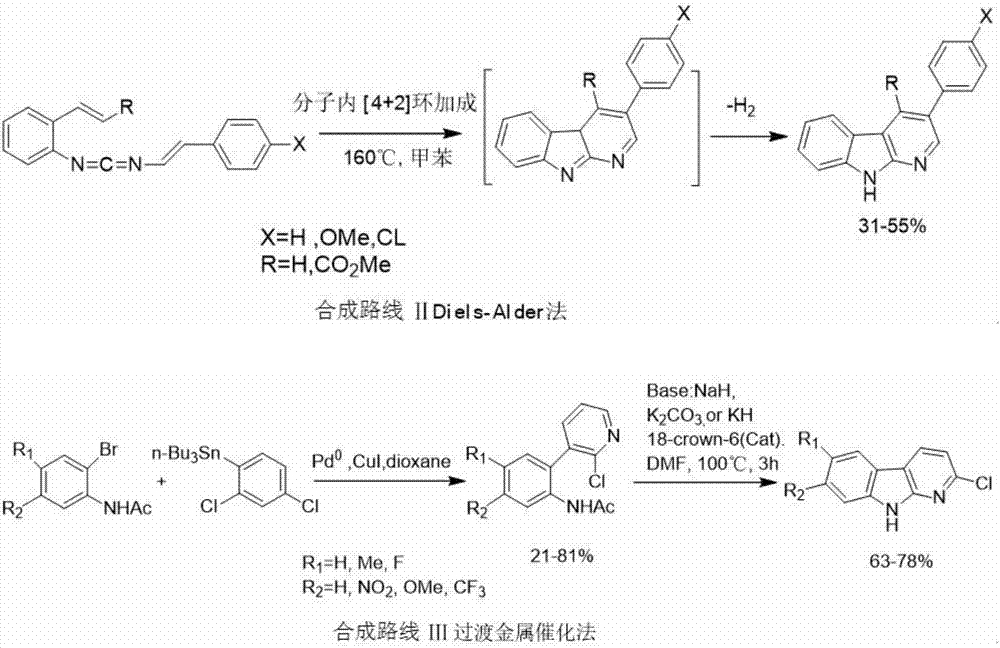
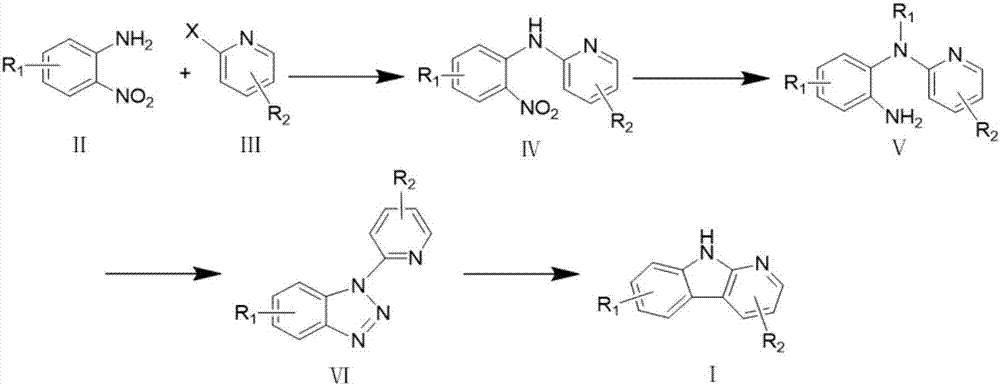
Synthesizing method of alpha-carboline compound
A synthesis method and compound technology, applied in the field of synthesis of α-carbolins, can solve the problems of difficult preparation or acquisition of starting materials, harsh reaction conditions, lack of flexibility, etc., to achieve increased yield and mild reaction conditions , the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Taking the preparation of 2-methyl-3-bromo-9H-pyridin[2,3-b]indole as an example
[0053] The synthesis process steps are: (1) Dissolve the compound 2,5-dibromo-6-picoline (2.70g, 10.76mmol) and o-nitroaniline (1.78g, 12.91mmol) in DMF (30mL), add t -BuOK (3.62g, 32.28mmol), 5%Pd / C (474.68mg, 2.96mmol), evacuate air and protect with nitrogen, stir the mixture at 120°C for 5h, cool, add 20mL water to dissolve, extract with EtOAc, The extract was washed successively with water and saturated brine, anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and purify the residue by silica gel column chromatography to obtain the product 2-(2-nitroanilino)-5-bromo-6-methylpyridine with a yield of 65%;
[0054] (2) Dissolve the compound 2-(2-nitroanilino)-5-bromo-6-methylpyridine (2.41g, 6.8mmol) in EtOAc (20mL), add SnCl 2 (6.46g, 34.08mmol), the mixture was stirred and refluxed at 80°C for 1.5h, cooled, dissolved in 20mL of water, and dissolved in K 2 CO 3...
Embodiment 2
[0058] Step (1) The reaction temperature is 100°C, the reaction time is 10h, the reaction solvent is DMSO, CuI is the catalyst, and t-BuONa is the acid scavenger to obtain 2-(2-nitrophenylamino)-5-bromo-6-methyl The yield of pyridine was 64.7%.
[0059] Step (2) The reaction temperature is 100°C, the reaction time is 2h, the reaction solvent is tetrahydrofuran, iron powder-ammonium chloride is a reducing agent, and 2-(2-aminophenylamino)-5-bromo-6-picoline is obtained. The yield was 91%.
[0060] Step (3) The reaction temperature is -10°C, the reaction time is 3h, the solvent is propanol, and concentrated HCl is changed to hyposulfuric acid to obtain 1-(5-bromo-6-methyl-2-pyridyl)-1H-benzene The yield of triazole was 96.1%.
[0061] Step (4) The reaction temperature is 180°C, the reaction time is 0.5h, the polyphosphoric acid is changed to pyrophosphoric acid, and the yield of 2-methyl-3-bromo-9H-pyridine[2,3-b]indole is 53.7% .
[0062] Other steps are with embodiment 1. ...
Embodiment 3
[0064] Step (1) The reaction temperature is 140°C, the reaction time is 1h, the reaction solvent is toluene, Ni is the catalyst, and t-BuONa is the acid scavenger to obtain 2-(2-nitrophenylamino)-5-bromo-6-methyl The yield of pyridine was 62.8%.
[0065] Step (2) The reaction temperature is 100°C, the reaction time is 2h, the reaction solvent is tetrahydrofuran, iron powder-ammonium chloride is a reducing agent, and 2-(2-aminoanilino)-5-bromo-6-methylpyridine is obtained. The yield was 91%.
[0066] Step (3) The reaction temperature is -10°C, the reaction time is 3h, the solvent is propanol, and concentrated HCl is changed to hyposulfuric acid to obtain 1-(5-bromo-6-methyl-2-pyridyl)-1H-benzene The yield of triazole was 96.1%.
[0067] Step (4) The reaction temperature was 100°C, the reaction time was 1 h, and the polyphosphoric acid was changed to pyrophosphoric acid to obtain 2-methyl-3-bromo-9H-pyridin[2,3-b]indole in a yield of 64.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com