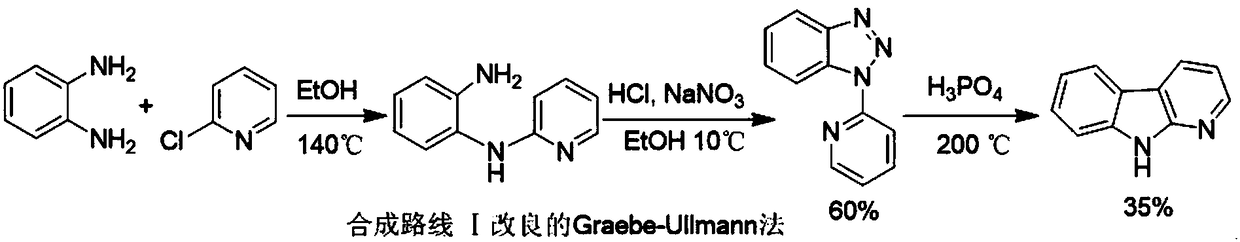
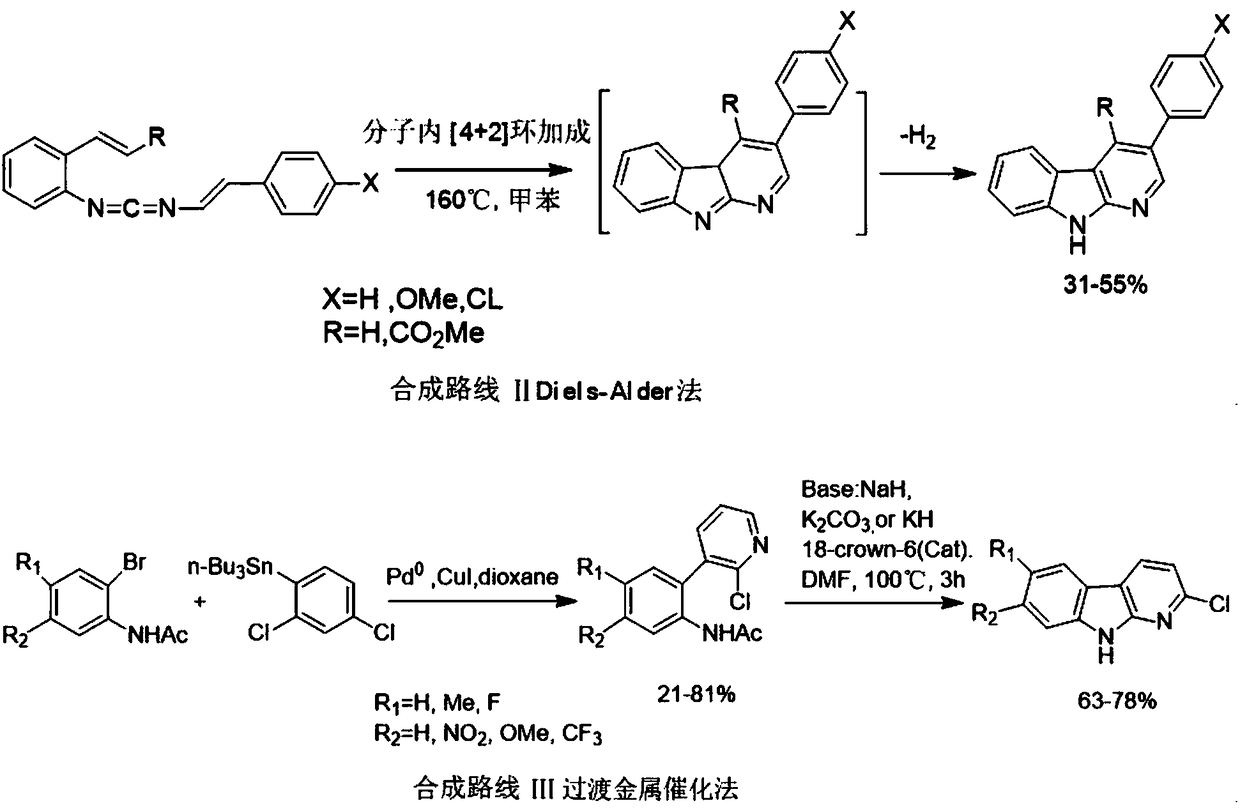
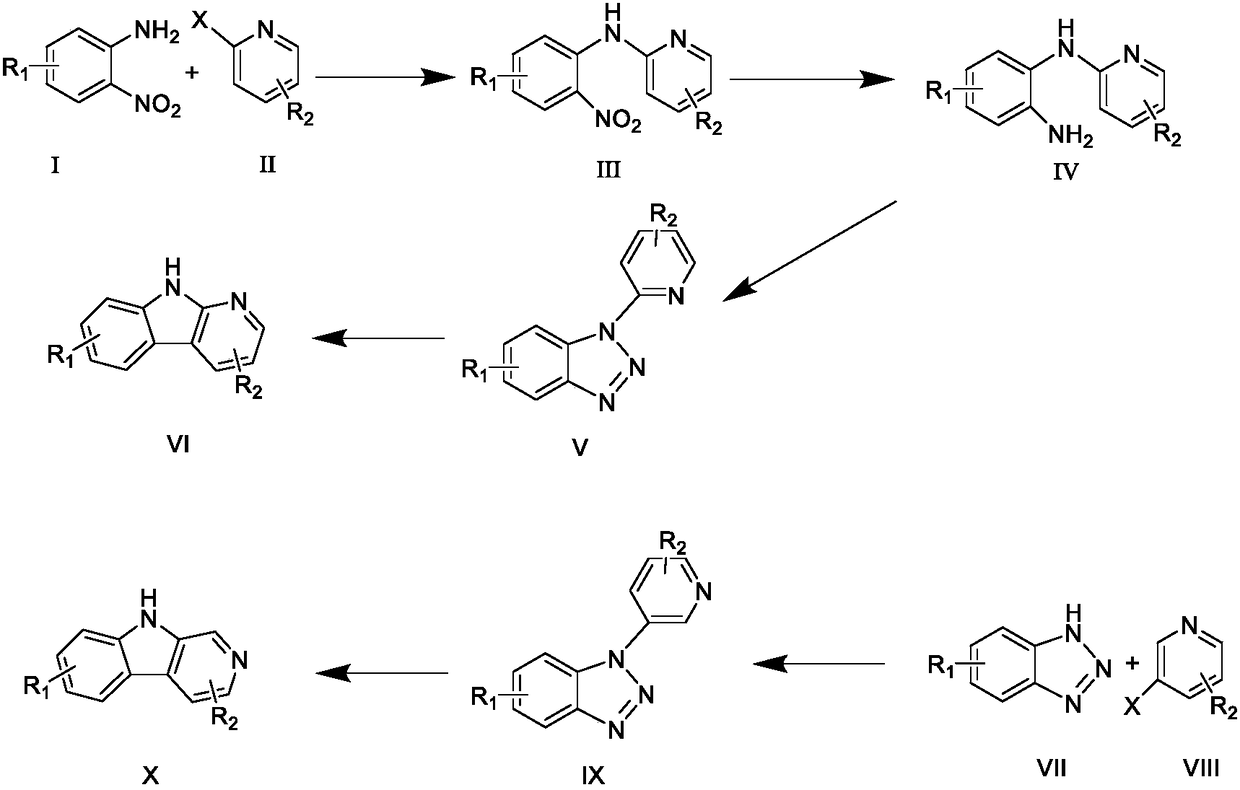
Synthesizing method of carbolin compound
A synthetic method and compound technology, applied in the field of synthesis of carboline compounds, can solve the problems of expensive reagents, high toxicity, difficult preparation or acquisition of starting materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Taking the preparation of 2-methyl-3-bromo-9H-pyridin[2,3-b]indole as an example
[0073] The synthesis process steps are: (1) Dissolve the compound 2,5-dibromo-6-picoline (2.70g, 10.76mmol) and o-nitroaniline (1.78g, 12.91mmol) in DMF (30mL), add t -BuOK (3.62g, 32.28mmol), 5%Pd / C (474.68mg, 2.96mmol), evacuate air and protect with nitrogen, stir the mixture at 120°C for 5h, cool, add 20mL water to dissolve, extract with EtOAc, The extract was washed successively with water and saturated brine, anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and purify the residue by silica gel column chromatography to obtain the product 5-bromo-o-nitroanilino-6-picoline with a yield of 66%;
[0074] (2) Dissolve 5-bromo-o-nitroanilino-6-picoline (2.41g, 6.8mmol) in EtOAc (20mL), add SnCl 2 (6.46g, 34.08mmol), the mixture was stirred and refluxed at 80°C for 3h, cooled, dissolved in 20mL of water, and dissolved in K 2 CO 3 Adjust PH=9, filter, and extract t...
Embodiment 1-1
[0078] In the aforementioned step (1), the mass ratio of 2,5-dibromo-6 picoline to o-nitroaniline is selected to be 1:1, 2:1, 3:1; the volumetric mass of solvent DMF and o-nitroaniline The ratio is 15:1, 16:1, 17:1, 18:1; the molar ratio of 5% Pd / C to o-nitroaniline is 0.2:1, 0.3:1, 0.4:1, 0.5:1; t- The molar ratio of BuOK to o-nitroaniline is 2:1, 3:1, 4:1. The product 5-bromo-o-nitroaniline-6-picoline was obtained, and the average yield was 64.8%. The yield was 42.5%, which was not ideal.
Embodiment 1-2
[0080] In the foregoing step (2), the volume (mL) molar ratio of EtOAc and 5-bromo-o-nitroanilino-6-picoline is selected to be 20:1, 30:1, 40:1; iron powder-chlorination The molar ratios of amine (1:3) to 5-bromo-o-nitroanilino-6-picoline are 4.5:1, 5:1, 5.5:1, 6:1. The average yield of the product 5-bromo-o-aminoanilino-6-picoline is more than 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com