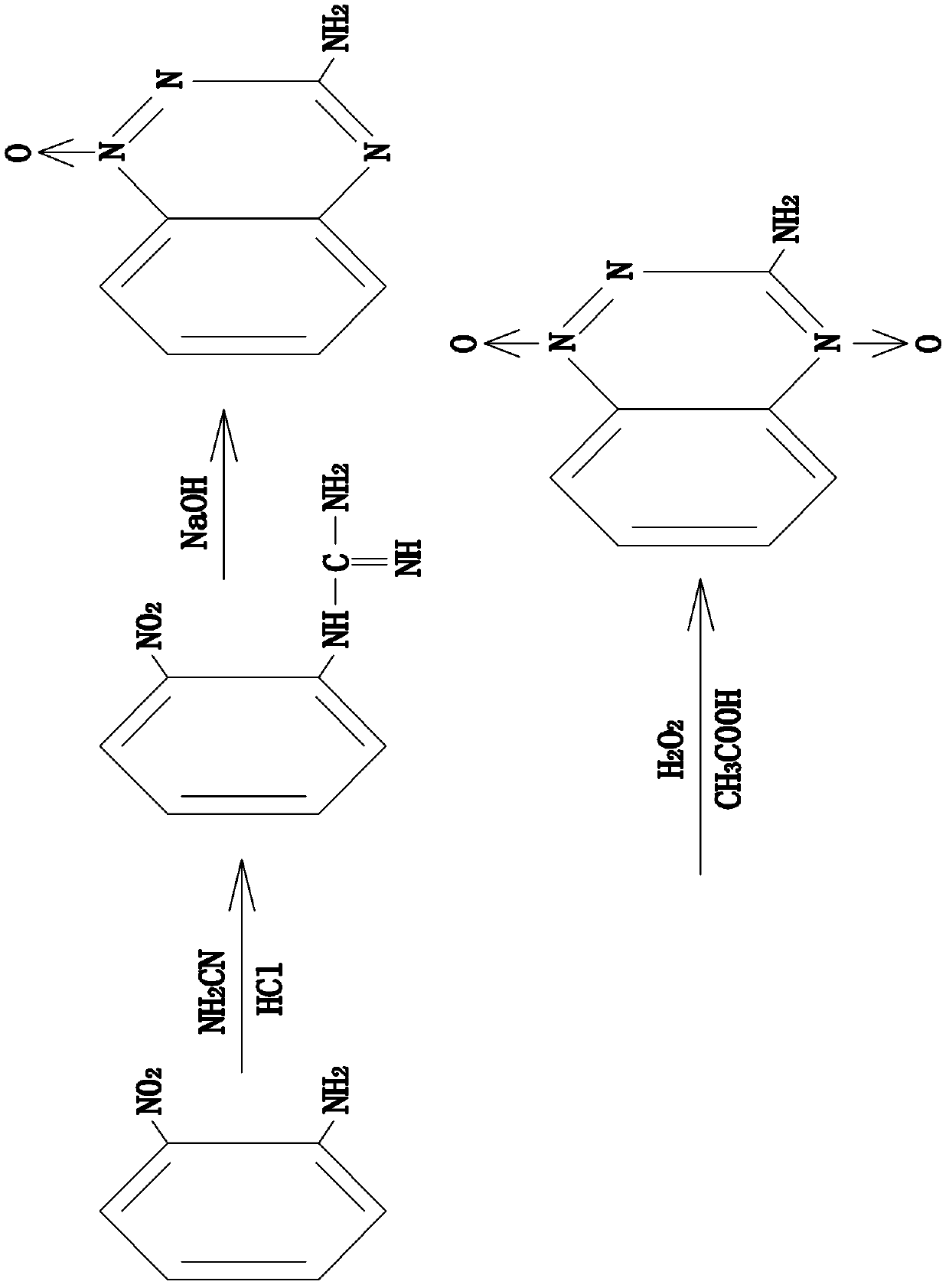
Synthesis method for 3-amino-1,2,4-phentriazine-1,4-dioxide
A technology of benzotriazine and dioxide, which is applied in the field of synthesis of 3-amino-1,2,4-benzotriazine-1,4-dioxide, can solve the problem of harsh reaction conditions, high toxicity, Unstable yield and other problems, to achieve the effect of mild reaction conditions, simple operation and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0017] 1) Weigh 15g of o-nitroaniline and stir and raise it to 50°C, slowly add 18mL of 55% cyanamide solution dropwise, after the dropwise addition, heat up to 100°C, continue heating until the solution turns dark red; down to room temperature, orange Yellow solid precipitated out, add 12mol / L concentrated HCl 60mL dropwise, drip in 20 minutes; heat up again to 100℃, stir for 20-40 minutes, then cool to room temperature; add 16mol / L NaOH solution 60mL; After reacting for 15 hours at 100°C, a viscous solid was suspended. Stir slowly; add 100-200 mL of water, stir, and cool to room temperature. A yellow solid precipitated, filtered, washed with water, washed with ethyl acetate, and dried to obtain a pale yellow powder. -Amino-1,2,4-benzotriazine-1-oxide.
[0018] In this step, the reaction time is sufficient, the concentration of the cyanamide solution is moderate, and 19.7 g of 3-amino-1,2,4-benzotriazine-1-oxide can be obtained, and the yield is 90%.
[0019] 2) Weigh 5g of 3-ami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com