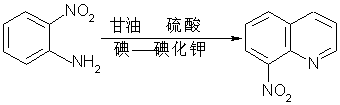
Synthetic method of 8-nitroquinoline
A technology of nitroquinoline and o-nitroaniline, applied in the direction of organic chemistry and the like, can solve the problems of high generation of 6-nitroquinoline, unsuitable for industrial production, large environmental pollution, etc., and it is easy to reach the end of the reaction and the end of the reaction. Control, phenomenal effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 550 grams of 98% sulfuric acid in a 2L four-necked flask equipped with a condenser, a thermometer, a dropping funnel, a stirrer and a gas absorption device.
[0024] Add 276 grams of o-nitroaniline in batches under a controlled temperature below 80°C, slowly and naturally raise the temperature from room temperature to 70°C-80°C, and continue stirring for 20-30 minutes until completely dissolved.
[0025] Control 70°C-80°C, add 645 grams of glycerin dropwise for about 90 minutes, and keep warm for 60-70 minutes at about 80°C after dropping.
[0026] Add dropwise a solution prepared by dissolving 2.5 grams of iodine and 2.5 grams of potassium iodide in 10 grams of water, and slowly heat up to 130°C after dropping the solution. After about half an hour, purple reflux begins and gas is released, and the gas is absorbed with lye.
[0027] The reaction at 130°C was naturally heated to 140°C for about 1 hour, the color of the reflux gradually changed to colorless, and the ...
Embodiment 2
[0031] In a 2L four-port container equipped with a condenser, a thermometer, a dropping funnel, a stirrer and a gas absorption device
[0032] Add 500 grams of 98% sulfuric acid in the flask.
[0033] Add 276 grams of o-nitroquinoline in batches under control below 90°C, slowly and naturally raise the temperature from room temperature to 80°C-90°C, and continue stirring for 20-30 minutes until completely dissolved.
[0034] Control 80°C-90°C, add 552 grams of glycerol dropwise for about 90 minutes, and keep warm for 30-40 minutes at about 90°C after dropping.
[0035] Add dropwise a solution prepared by dissolving 2 g of iodine and 2 g of potassium iodide in 10 g of water, and slowly raise the temperature to 130°C after dropping, and for about half an hour, purple reflux begins and gas is released, and the gas is absorbed with lye.
[0036] 130 ° C reaction naturally heated to 140 ° C for about 1 hour, the reflux color gradually became clear, and continued to keep warm for 30...
Embodiment 3
[0040] Add 600 grams of 98% sulfuric acid in a 2L four-necked flask equipped with a condenser, a thermometer, a dropping funnel, a stirrer and a gas absorption device.
[0041] Add 276 grams of o-nitroquinoline in batches at a temperature below 70°C, slowly and naturally raise the temperature from room temperature to 60°C-70°C, and continue stirring for 20-30 minutes until completely dissolved.
[0042] Control 60°C-70°C, add 690 grams of glycerin dropwise for about 90 minutes, and keep warm for 75-90 minutes at about 70°C after dropping.
[0043] Add dropwise a solution prepared by dissolving 3 grams of iodine and 3 grams of potassium iodide in water, and slowly raise the temperature to 120°C for about half an hour after dropping, the reaction will naturally heat up and gradually purple reflux and gas will be released, and the gas will be absorbed with lye.
[0044] The reaction at 120°C was naturally heated to 140°C for about 1.5 hours, the color of the reflux gradually beca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com