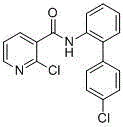
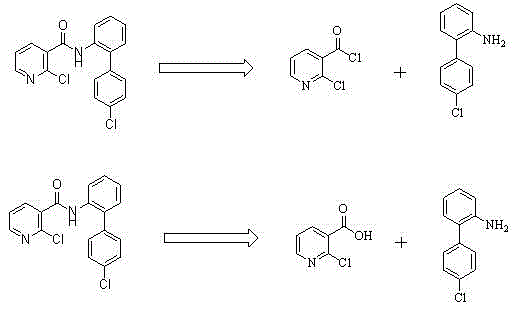
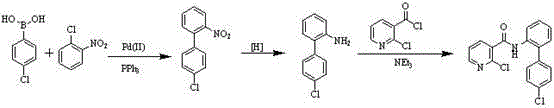
Preparation method of nicotinamide fungicide namely boscalid
A technology of boscalid and nicotinamide, which is applied in the field of preparation of nicotinamide broad-spectrum fungicide boscalid and its intermediates, which can solve the problem of unsatisfactory preparation discovery, high preparation cost, and many side reactions. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A preparation method of boscalid, a nicotinamide fungicide is provided, comprising the steps of:
[0051] (1) Preparation of p-chlorophenylboronic acid (compound II)
[0052] Under the protection of nitrogen, directly into the 20-liter reaction kettle, add in sequence: 0.55Kg of magnesium chips and 5.0Kg of 2-methyltetrahydrofuran. The temperature was raised to reflux, and 1Kg of 2-methyl THF solution of 3.80Kg of p-chlorobromobenzene was added dropwise. After the dropwise addition, the reflux reaction was maintained for 4-5h. After the reaction was complete, the reaction was stopped and cooled to room temperature. will (i-PrO) 3 B. Add 5.20 Kg of 4.0 Kg 2-methyltetrahydrofuran solution into a 50-liter reaction kettle, and add the above-prepared Grignard reagent dropwise at a temperature not higher than 0°C; Add 16L of 20% HCl, stir for 1h, add 5L of toluene, extract, and filter with suction; continue to stir and filter with 5L of toluene for the solid, combine the l...
Embodiment 2
[0062] A preparation method of boscalid, a nicotinamide fungicide is provided, comprising the steps of:
[0063] (1) Preparation of p-chlorophenylboronic acid (compound II)
[0064] Under the protection of nitrogen, directly into the 20-liter reaction kettle, add in sequence: 0.55Kg of magnesium chips and 5.0Kg of 2-methyltetrahydrofuran. The temperature was raised to reflux, and 1Kg of 2-methyl THF solution of 3.80Kg of p-chlorobromobenzene was added dropwise. After the dropwise addition, the reflux reaction was maintained for 4-5h, the reaction was stopped, and cooled to room temperature. Will (n-BuO) 3 B 6.00 Kg of 4.0 Kg 2-methyltetrahydrofuran solution was added to a 50-liter reaction kettle, and the Grignard reagent prepared above was added dropwise at a temperature not higher than 0°C; after the dropwise addition, stirred for 10 hours; then added 10% H 2 SO 4 16L, stirred for 1h, added 5L toluene, extracted, and suction filtered; the solid was continuously stirred...
Embodiment 3
[0071] A preparation method of boscalid, a nicotinamide fungicide is provided, comprising the steps of:
[0072] (1) Preparation of o-iodonitrobenzene (compound III)
[0073] N 2 Under protection, at room temperature, 1 Kg of o-nitroaniline and 10 L of concentrated hydrochloric acid were added to the above reaction kettle. The above solution was stirred and cooled until the temperature in the bottle reached below 0°C. Dissolve 0.53 Kg of sodium nitrite in 1.2 L of water to form a solution, slowly add it into the reaction kettle, and stir for 1 h. Under the condition of not higher than 0°C, dissolve 1.5 Kg NaI in 1L of water and add it to a new reaction kettle, and stir. The prepared diazonium solution was added dropwise to the above reaction solution, and after the dropwise addition was completed, the reaction was continued for 1 h. After the reaction, it was filtered, dried in the air, recrystallized with ethanol, cooled and precipitated, and filtered to obtain 1.35 Kg of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com