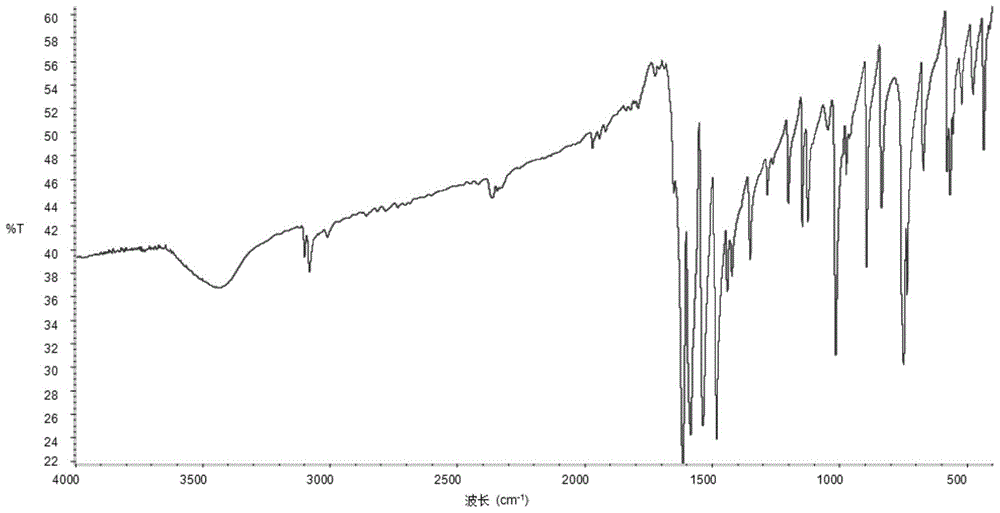
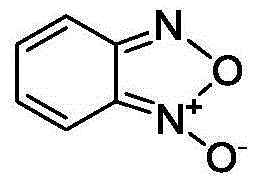
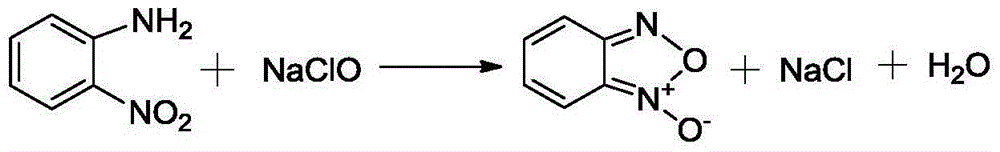
Method for preparing benzofuroxan under catalysis of surfactant micelle
A technology of benzofuroxan and surfactant, which is applied in the field of micellar catalytic oxidation in organic synthesis methods, can solve the problems of difficult crystallization of products, low product purity, incomplete reaction and the like, and achieves easy products, little environmental pollution, reaction to full effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] Concrete synthetic method comprises the following steps in turn:
[0024] (1) Add 0.1-1g surfactant, 1-2g KOH, 20-25ml water into a dry reactor, stir and mix evenly, then add 6-8g o-nitroaniline;
[0025] (2) Add 70-90ml sodium hypochlorite solution dropwise at 30-35°C, stir while adding dropwise, and continue to react for 2-4 hours after dropping;
[0026] (3) The reaction solution was filtered, and the solid was vacuum-dried at 30° C. to obtain a yellow solid, which was the crude product BFO;
[0027] (4) Wash the solid three times with water, and then recrystallize it with absolute ethanol. The amount of solvent used in the crystallization is 5 to 7 times the mass of the product. After heating and dissolving, place the solution in an environment of -10 to -25°C to cool and crystallize , to obtain yellow flaky crystals.
[0028] Surfactant micelles can catalyze the reaction of o-nitroaniline to prepare benzofurazan, but the catalytic effect varies with the types of ...
Embodiment 1
[0031] Example 1: Preparation of hydroxyl-containing cationic gemini surfactants for catalytic oxidation of BFO
[0032] Add 0.1g hydroxyl-containing cationic gemini surfactant, 1gKOH, and 20ml water into a dry reactor, stir and mix evenly, then add 6g o-nitroaniline, add 80ml sodium hypochlorite solution dropwise at 30~35°C, and add While stirring, the reaction continued for 2 hours after the drop was completed. After the reaction was completed, the reaction solution was filtered, and the solid was vacuum-dried at 30°C to obtain a yellow solid, which was the crude product BFO. The solid was washed with water, and then washed with absolute ethanol Recrystallized to obtain pure BFO yellow crystals. The yield was 97% and the purity was 96%.
Embodiment 2
[0033] Example 2: Preparation of BFO Catalyzed Oxidation by Anionic Gemini Surfactant
[0034] Add 0.5g of anionic gemini surfactant, 1gKOH, and 20ml of water into a dry reactor, stir and mix evenly, then add 6g of o-nitroaniline, add 80ml of sodium hypochlorite solution dropwise at 30-35°C, and stir while adding After the drop, the reaction was continued for 2 hours. After the reaction, the reaction solution was filtered, and the solid was dried in vacuum at 30°C to obtain a yellow solid, which was the crude product BFO. The solid was washed with water, and then recrystallized with absolute ethanol , to obtain pure BFO yellow crystals. The yield was 92% and the purity was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com