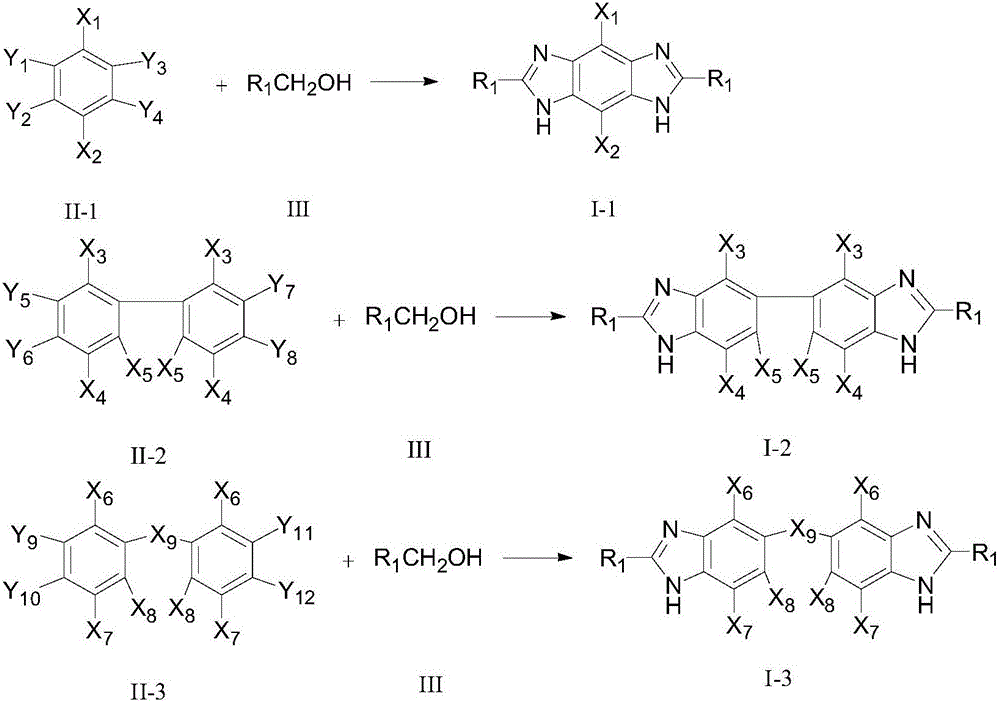
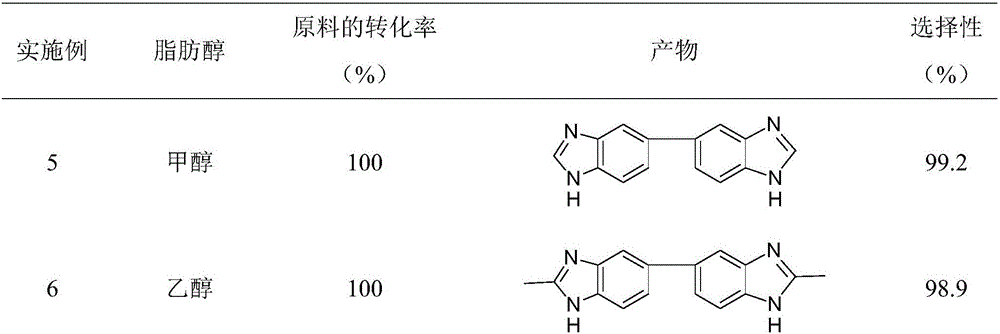
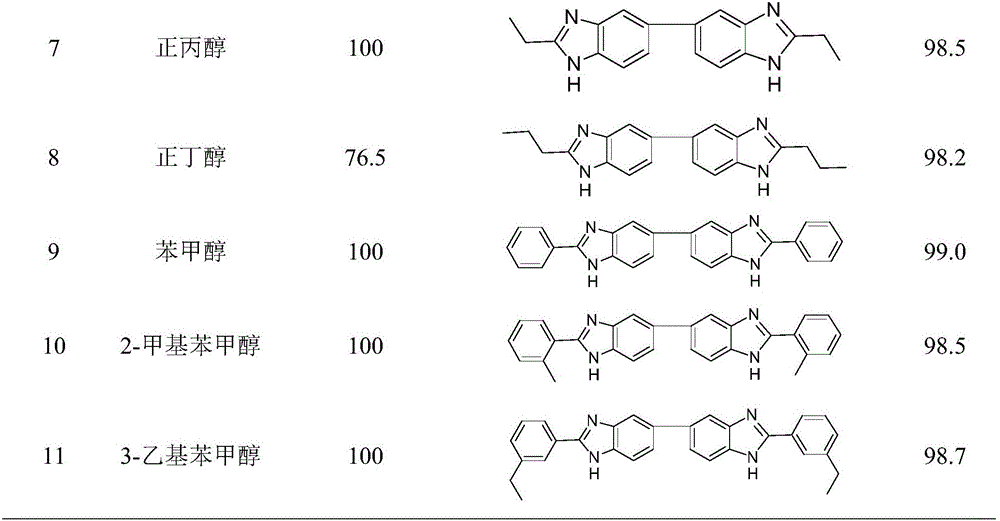
Method for synthesizing bis-benzimidazole compound through one-pot method
A technology for bisbenzimidazole and compound is applied in the field of synthesizing bisbenzimidazole compounds, and can solve the problems of inapplicable bisbenzimidazoles, low yield, poor activity and selectivity of bisbenzimidazoles, etc. To achieve the effect of convenient product separation, convenient industrial production and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 10g specific surface area as 250m 2 g -1 , average pore size 8nm, particle size 500 mesh γ-Al 2 o 3 Add water and stir, heat in a water bath to 60°C, add 10mL of H 2 PdCl 4 solution (Pd content is 0.05g / mL), 10mLMg(NO 3 ) 2 solution (Mg content is 0.03g / mL) and 10mL Cu(NO 3 ) 2 Solution (Cu content is 0.05g / mL), kept at 60°C for 4 hours, added dropwise NaOH solution to adjust the pH value to 8-10, kept the solution temperature and stirred for 1 hour; filtered, and the filter cake was washed with deionized water to medium properties; vacuum drying at 100°C for 5h; then, calcining at 300°C for 4h, and finally reducing with hydrogen at 300°C for 4h to obtain Cu5wt%-Mg3wt%-Pd5wt% / γ-Al 2 o 3 catalyst.
Embodiment 2
[0035] Take 10g specific surface area as 350m 2 g -1 , average pore size 10nm, particle size 800 mesh γ-Al 2 o 3 Add water and stir, heat in a water bath to 80°C, add dropwise 10mL of Pt(NO 3 ) 2 solution (Pt content is 0.08g / mL), 10mLBaCl 2 solution (Ba content is 0.02g / mL) and 10mL CuCl 2 Solution (Cu content is 0.02g / mL), kept at 80 ℃ for 4 hours, added dropwise (NH 4 ) 2 CO 3 Adjust the pH value of the solution to between 8 and 10, keep the solution temperature and stir for 3 hours; filter, wash the filter cake with deionized water until neutral; vacuum dry at 105°C for 4h; then, roast at 260°C for 8h, and finally in Reduction with hydrogen at 300°C for 5 hours to obtain Cu2wt%-Ba2wt%-Pt8wt% / γ-Al with corresponding loading 2 o 3 catalyst.
Embodiment 3
[0037] Take 10g specific surface area as 450m 2 g -1 , average pore size 20nm, particle size 800 mesh γ-Al 2 o 3 Add water and stir, heat in a water bath to 90°C, add 10mL of H 2 PtCl 6 solution (Pt content is 0.03g / mL), 10mL Pd(C 2 h 3 o 2 ) 2 Solution (Pd content is 0.03g / mL), 10mLKNO 3 solution (K content is 0.04g / mL) and 10mL Cu(NO 3 ) 2 Solution (Cu content: 0.03g / mL), kept at 90°C for 8 hours, added KOH solution dropwise to adjust the pH value to 8-10, kept the solution temperature and stirred for another 2 hours; filtered, and the filter cake was washed with deionized water to medium properties; vacuum drying at 110°C for 8h; then calcination at 400°C for 5h, and finally reduction with hydrogen at 350°C for 3h to obtain Cu3wt%-K4wt%-Pd3wt%-Pt3wt% / γ-Al 2 o 3 catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com