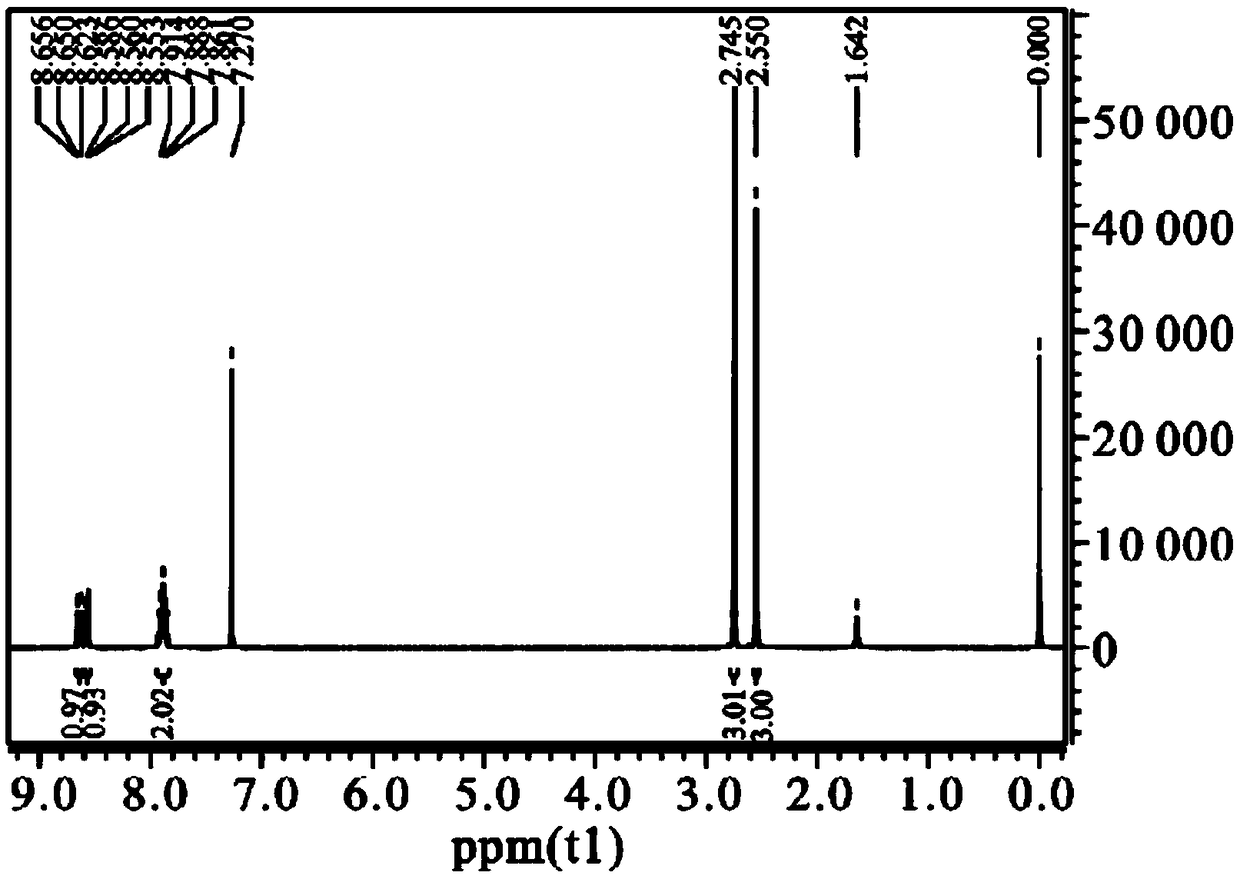
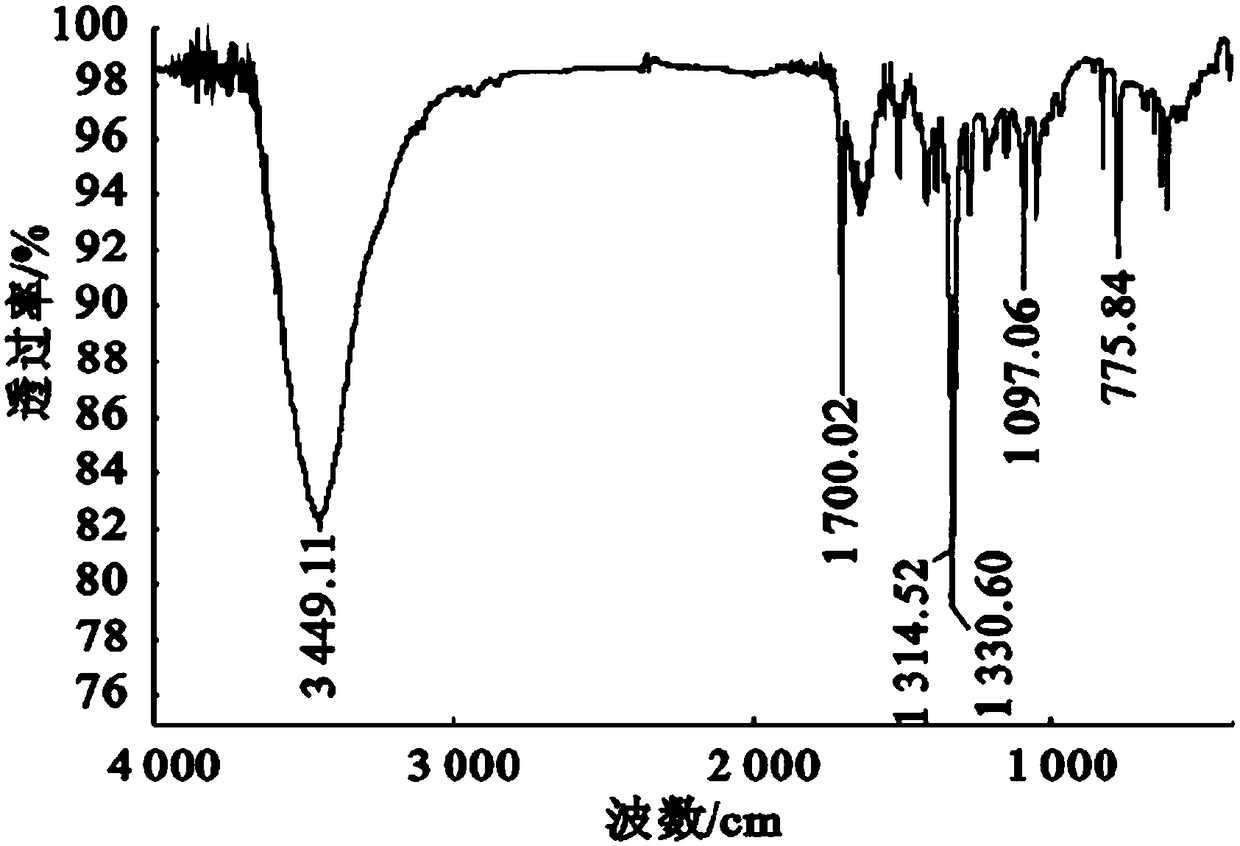
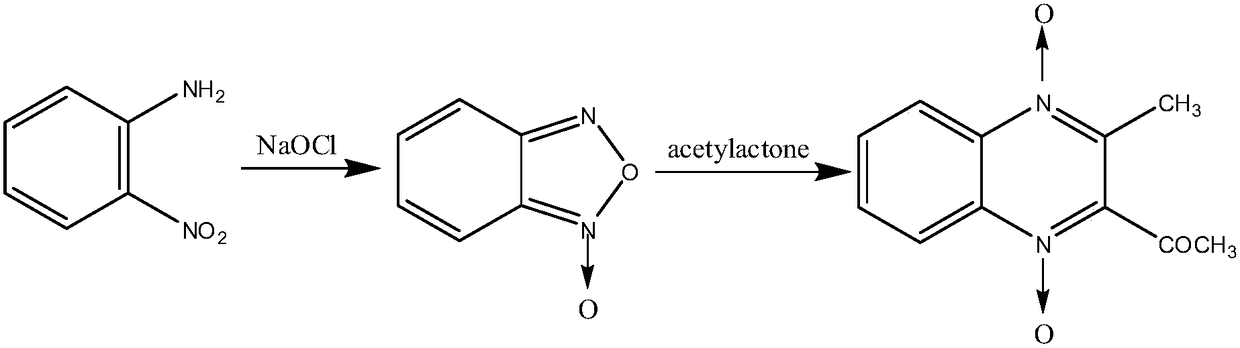
Preparation method of mequindox
A technology of acemequine and acetylacetone, applied in the field of preparation of acemequine, can solve the problems of low product yield and purity, many steps, long reaction time and the like, and achieve the effects of improving purity, reducing loss and reducing reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of preparation method of acetylmethaquine:
[0048] Add 920ml of methanol and 91.08g (0.66mol) of o-nitroaniline in a 500mL three-necked flask equipped with electromagnetic stirring, reflux condenser and thermometer, add 40.99g of NaOH / attapulgite compound to it, and add carboxymethyl fiber Add 18.22 g of plain sodium, stir and heat at 30°C to make it completely dissolve. Then, 68.31 g of sodium hypochlorite (containing 5% available chlorine) was slowly added dropwise, the temperature was controlled at 30° C., and the dropwise addition was completed within 3 hours. Keep warm at 30°C for 2 hours to complete the reaction.
[0049] Add 97ml (0.9372mol) of acetylacetone, stir to dissolve it, keep it warm at 35°C for 8h, place it at room temperature for 12h, wash out a lot of solids, filter, and recrystallize twice with ethanol to get yellow needle crystals, 120°C After drying for 2 hours, acemetquine was obtained with a total yield of 84.5%. The melting point is 1...
Embodiment 2
[0054] A kind of preparation method of acetylmethaquine:
[0055]Add 575ml of methanol and 91.08g (0.66mol) of o-nitroaniline in a 500mL three-necked flask equipped with electromagnetic stirring, reflux condenser and thermometer, and add 45.54g of NaOH / attapulgite compound to it, and then carboxymethyl fiber Add 22.77 g of plain sodium, stir and heat at 25°C to make it completely dissolve. Then, 72.86 g of sodium hypochlorite (containing 5% available chlorine) was slowly added dropwise, the temperature was controlled at 25° C., and the dropwise addition was completed within 3 hours. Keep warm at 25°C for 2.5 hours to complete the reaction.
[0056] Add 68ml (0.66mol) of acetylacetone, stir to dissolve it, keep it warm at 25°C for 20h, place it at room temperature for 12h, wash out a large amount of solids, filter, and recrystallize twice with ethanol to obtain yellow needle crystals, 120°C After drying for 2 hours, acemetquine was obtained with a total yield of 78.9%. The m...
Embodiment 3
[0060] A kind of preparation method of acetylmethaquine:
[0061] Add 1150ml of methanol and 91.08g (0.66mol) of o-nitroaniline in a 500mL three-necked flask equipped with electromagnetic stirring, reflux condenser and thermometer, and add 27.32g of NaOH / attapulgite compound to it, and add carboxymethyl fiber Add 13.66 g of plain sodium, stir and heat at 35°C to dissolve it completely. Then, 63.76 g of sodium hypochlorite (containing 5% available chlorine) was slowly added dropwise, the temperature was controlled at 35° C., and the dropwise addition was completed within 3 hours. Keep warm at 35°C for 2 hours to complete the reaction.
[0062] Add 116ml (1.122mol) of acetylacetone, stir to dissolve it, incubate at 40°C for 2h, place at room temperature for 12h, wash out a large amount of solids, filter, and recrystallize twice with ethanol to obtain yellow needle crystals, dry at 120°C After 2h, acemetquine was obtained with a total yield of 71.8%. The melting point is 152-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com