Method for synthesizing benzimidazole compounds
A technology for benzimidazoles and compounds is applied in the field of one-step synthesis of benzimidazole compounds, which can solve the problems of troublesome product separation and many reaction steps, achieve convenient product separation, reduce the generation of by-products, and avoid strong oxidants and strong acid solvents. the effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
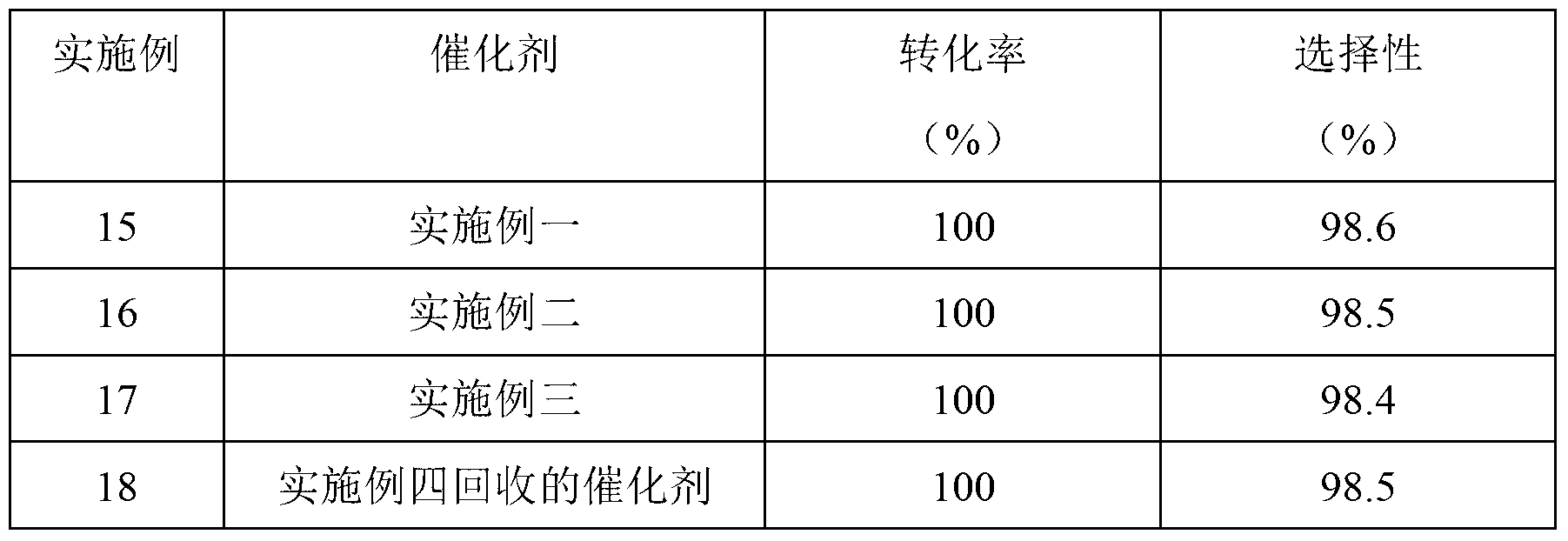
Embodiment 1
[0030] Take 10g Al 2 o 3 Add water and stir, heat in a water bath to 80°C, add 10mL of H 2 PdCl 4 solution (Pd content is 0.03g / mL), 10mL Zn(NO 3 ) 2 solution (Zn content is 0.02g / mL) and 10mL Cu(NO 3 ) 2 solution (Cu content is 0.04g / mL), kept at 80°C for 5 hours, then added dropwise NH 4 HCO 3 Adjust the pH value of the solution to 8-10, keep the solution temperature and stir for 1 hour; filter, wash the filter cake with deionized water until neutral; vacuum dry at 110°C for 4 hours; then, roast at 260°C for 4 hours, and finally in Reduction with hydrogen at 260°C for 4 hours, that is, Cu4wt%-Zn2wt%-Pd3wt% / Al with the corresponding loading amount can be obtained 2 o 3 catalyst.
Embodiment 2
[0032] Take 10g Al 2 o 3 Add water and stir, heat in a water bath to 80°C, add dropwise 20mL of Pd(NO 3 ) 2 solution (Pd content is 0.01g / mL), 5mL ZnCl 2 solution (Zn content is 0.02g / mL) and 5mL CuCl 2 solution (Cu content is 0.1g / mL), kept at 70°C for 3 hours, then added dropwise (NH 4 ) 2 CO 3 Adjust the pH value of the solution to 8-10, keep the solution temperature and stir for 4 hours; filter, wash the filter cake with deionized water until neutral; vacuum dry at 90°C for 6h; then, roast at 360°C for 5h, and finally in Reduction with hydrogen at 300°C for 3 hours, that is, Cu5wt%-Zn1wt%-Pd2wt% / Al with corresponding loads 2 o 3 catalyst.
Embodiment 3
[0034] Take 10g Al 2 o 3 Add water and stir, heat in a water bath to 80°C, add dropwise 30mL of Pd(C 2 h 3 o 2 ) 2 solution (Pd content is 0.01g / mL), 5mL Zn(NO 3 ) 2 solution (Zn content is 0.01g / mL) and 10mL Cu(NO 3 ) 2 solution (Cu content is 0.02g / mL), kept at 90°C for 8 hours, added dropwise NaOH solution to adjust the pH value to 8-10, kept the solution temperature and stirred for 2 hours; filtered, and the filter cake was washed with deionized water until medium properties; vacuum drying at 100°C for 8h; then calcination at 400°C for 5h, and finally, hydrogen reduction at 350°C for 3h to obtain Cu2wt%-Zn0.5wt%-Pd3wt% / Al 2 o 3 catalyst.
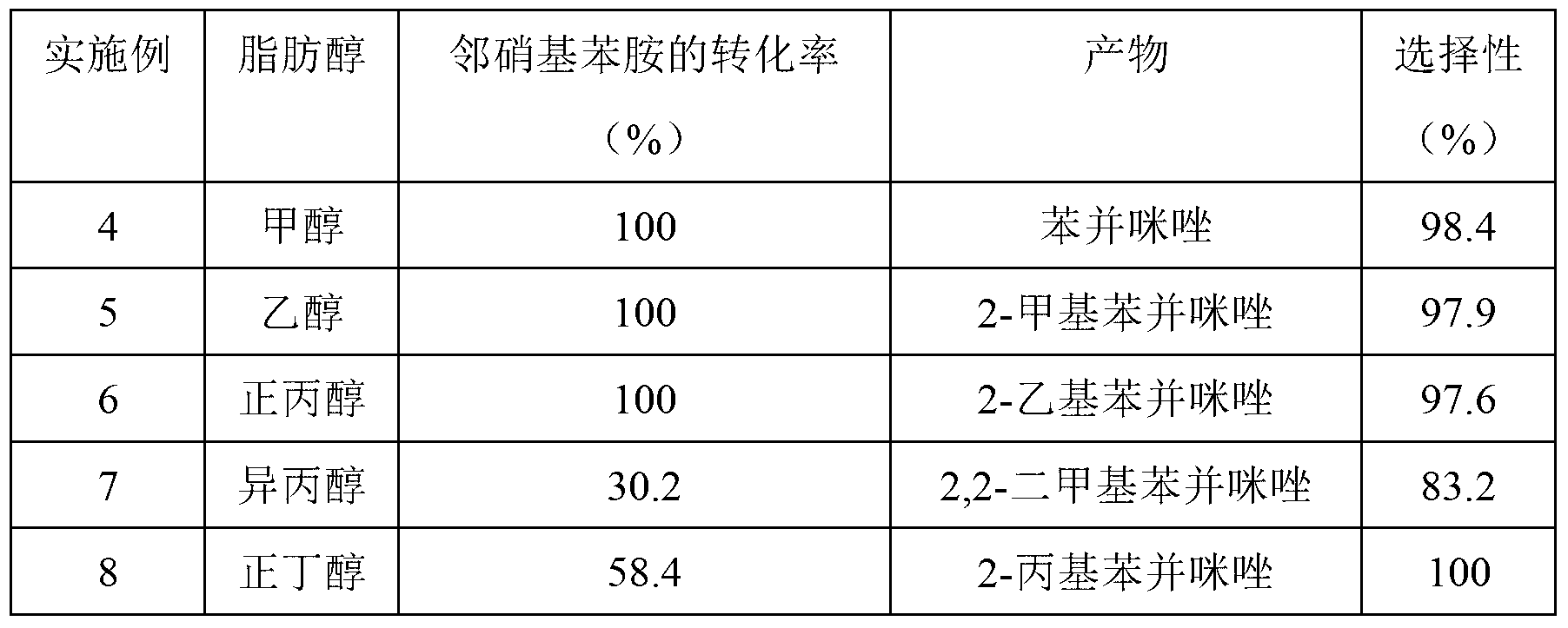
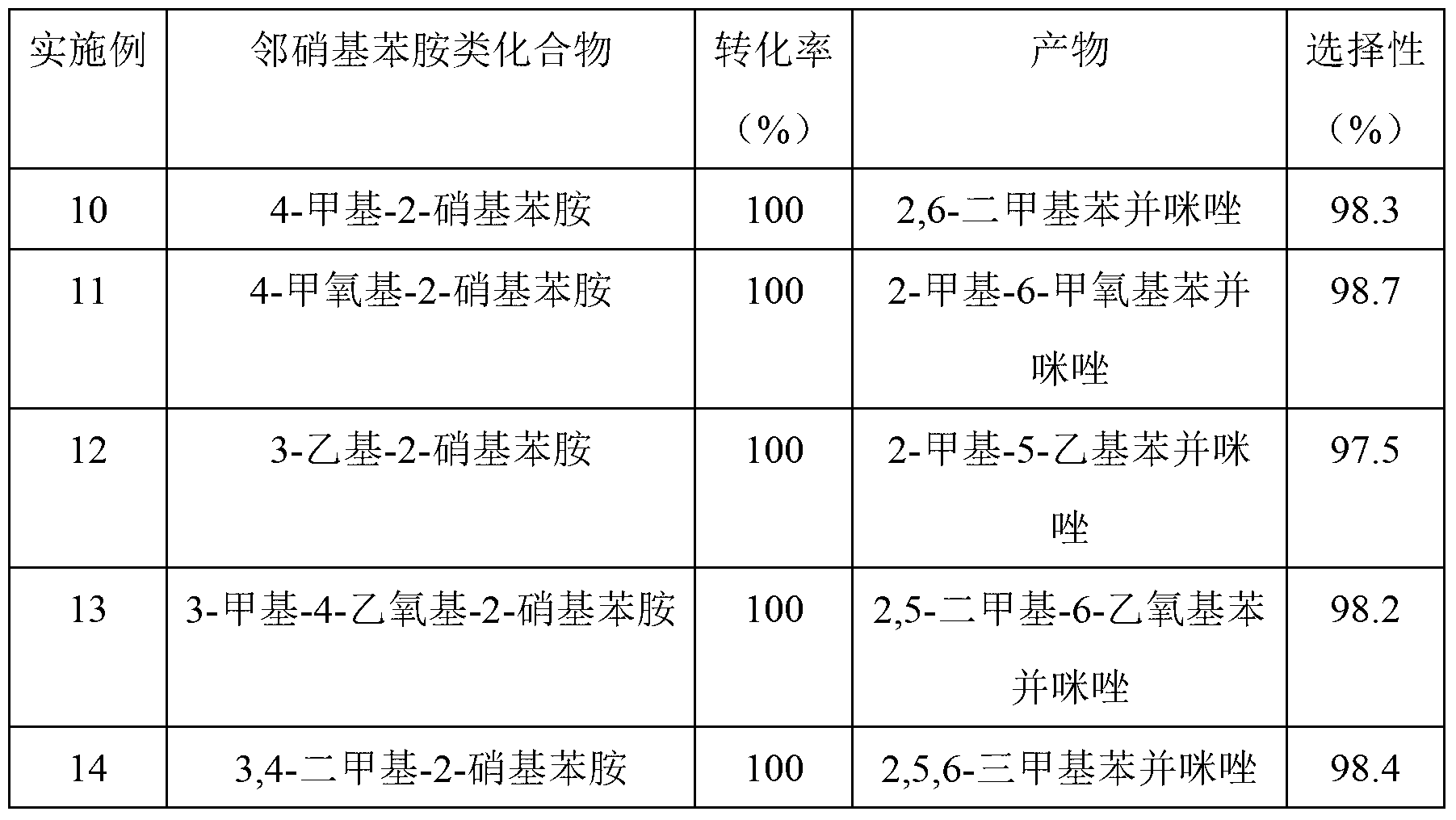
[0035] Embodiment 4 to Embodiment 12 are Cu-Zn-Pd / Al prepared by the above method 2 o 3 Examples of catalysts used in the synthesis of benzimidazoles:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com