Benzoxazole and benzimidazole compounds and preparation method thereof
A technology of benzimidazole and benzoxazole, which is applied in the field of benzoxazole and benzimidazole compounds and their preparation, can solve the problems of complex preparation process, high price, and increased reaction cost, and achieve simple operation and low cost. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation method of described a kind of benzoxazole and benzimidazole compound, comprises the following steps:
[0046] a) amino acid with structure (I) and o-nitrophenol (o-nitroaniline) compound with structure (II) can be obtained with structure (III) by stirring and heating in a solvent under the catalysis of a base Benzoxazole and benzimidazole compounds of the present invention:
[0047]
[0048] Among them, R 1 is aryl, substituted aryl or alkyl; R 2 is fluorine, chlorine, methoxy or methyl; X is oxygen, nitrogen or nitrogen methyl.
[0049] R 1 Is aryl, said aryl is phenyl or naphthyl; said R 1 is a substituted aryl group, and the substituted aryl group is p-chlorophenyl, p-methylphenyl, 3,4-dimethoxyphenyl, p-methoxyphenyl, m-methoxyphenyl, o-methoxyphenyl Oxyphenyl, p-trifluoromethylphenyl, p-tert-butylphenyl, p-fluorophenyl, ethyl p-formate phenyl or p-bromophenyl; the R 1 is an alkyl group, and the alkyl group is methyl, n-propyl, tert-butyl or...
Embodiment 1
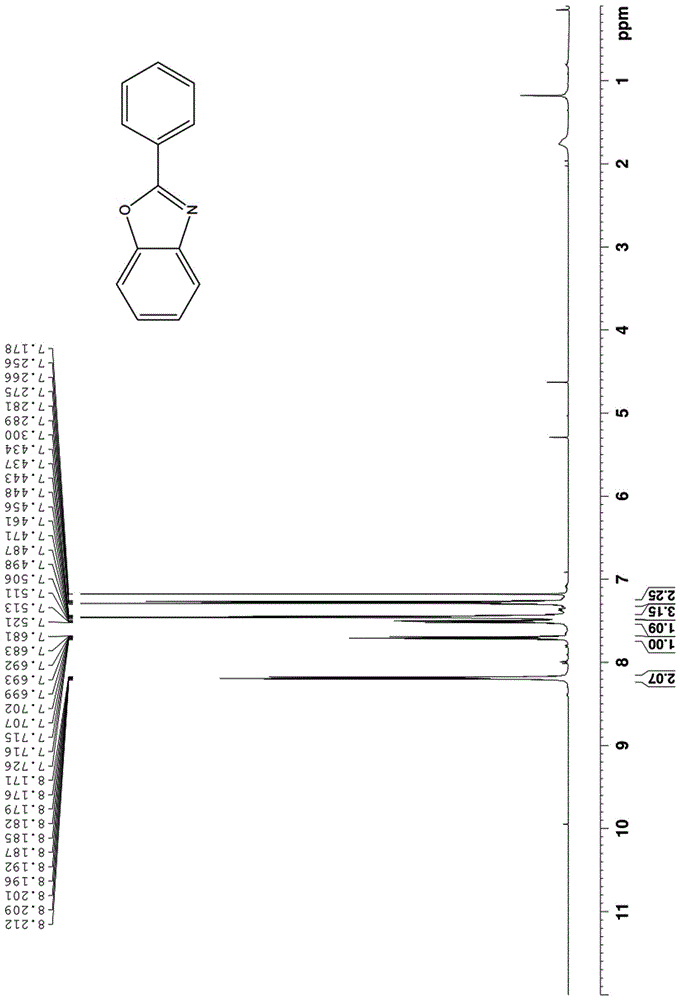
[0058] In a clean and dry 10 ml Schlenk reaction tube, add 35 mg of o-nitrophenol, 113 mg of phenylglycine, and 69 mg of potassium carbonate in sequence, use 1 ml of toluene as a solvent, seal the reaction tube, and react at 130°C for 24 hours . After the reaction, the reaction mixture was directly spin-dried by a rotary evaporator, and then separated by a silica gel column using petroleum ether and ethyl acetate at a volume ratio of 30:1 as eluents to obtain 46 mg of a white solid with a yield of 95%.
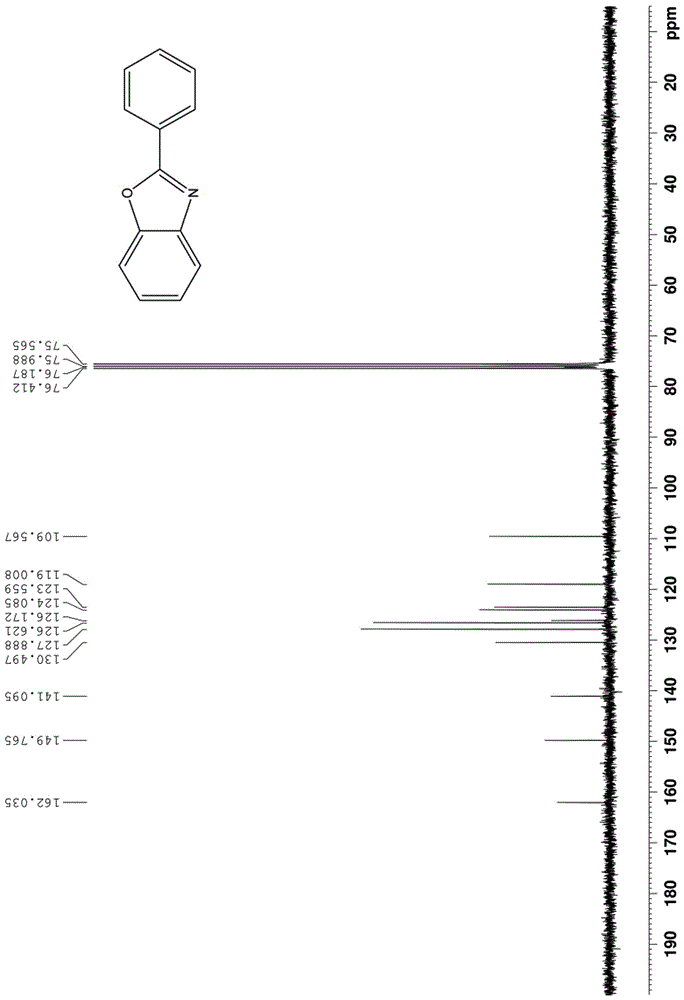
[0059] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the carbon NMR spectrum is as Figure 1b shown. It can be confirmed from the spectrum that the obtained product is 2-phenylbenzoxazole.
Embodiment 2
[0061] In a clean and dry 100 ml Schlenk reaction tube, add 700 mg of o-nitrophenol, 2260 mg of phenylglycine, and 1680 mg of potassium tert-butoxide in sequence, use 30 ml of toluene as solvent, seal the reaction tube, and react at 130°C 24 hours. After the reaction, the reaction mixture was directly spin-dried by a rotary evaporator, and then separated by a silica gel column using petroleum ether and ethyl acetate at a volume ratio of 30:1 as eluents to obtain 880 mg of a white solid with a yield of 90%.
[0062] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the carbon NMR spectrum is as Figure 1b shown. It can be confirmed from the spectrum that the obtained product is 2-phenylbenzoxazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com