Method for synthesizing azophenylene amide compound with antineoplastic activity and application of azophenylene amide compound
A technology of anti-tumor activity and phenazinamide, which is applied in the field of synthesis of phenazine amide compounds, can solve the problems of high toxicity and side effects of anti-tumor drugs, and achieve the effects of safe clinical medication, easy preparation method, and convenient source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
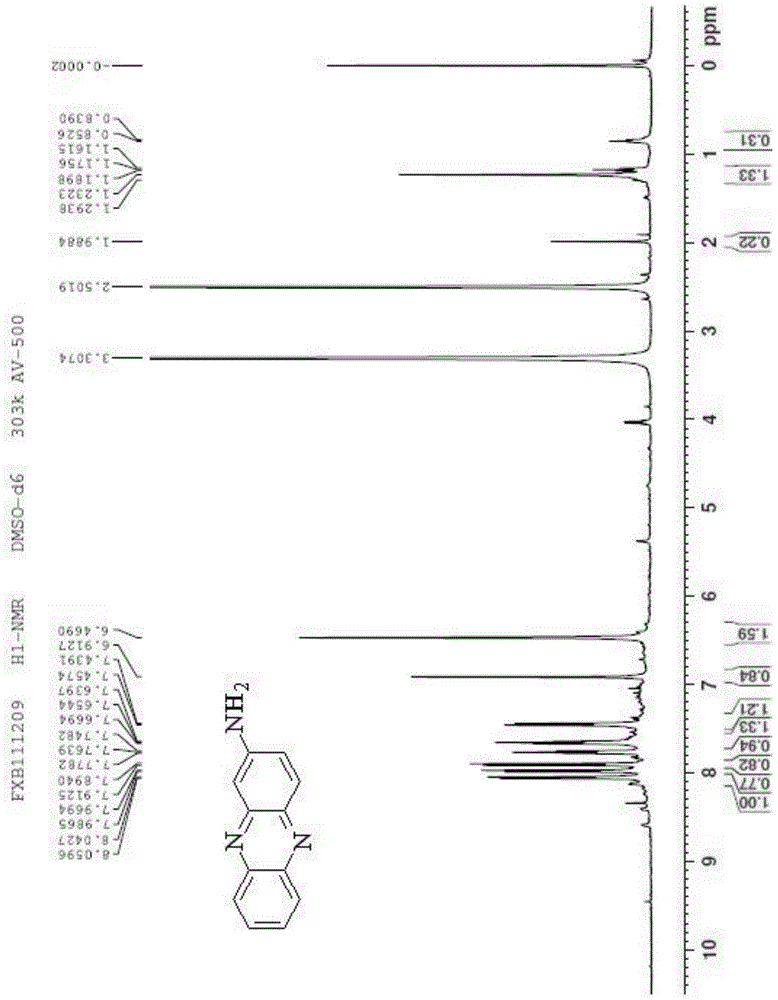
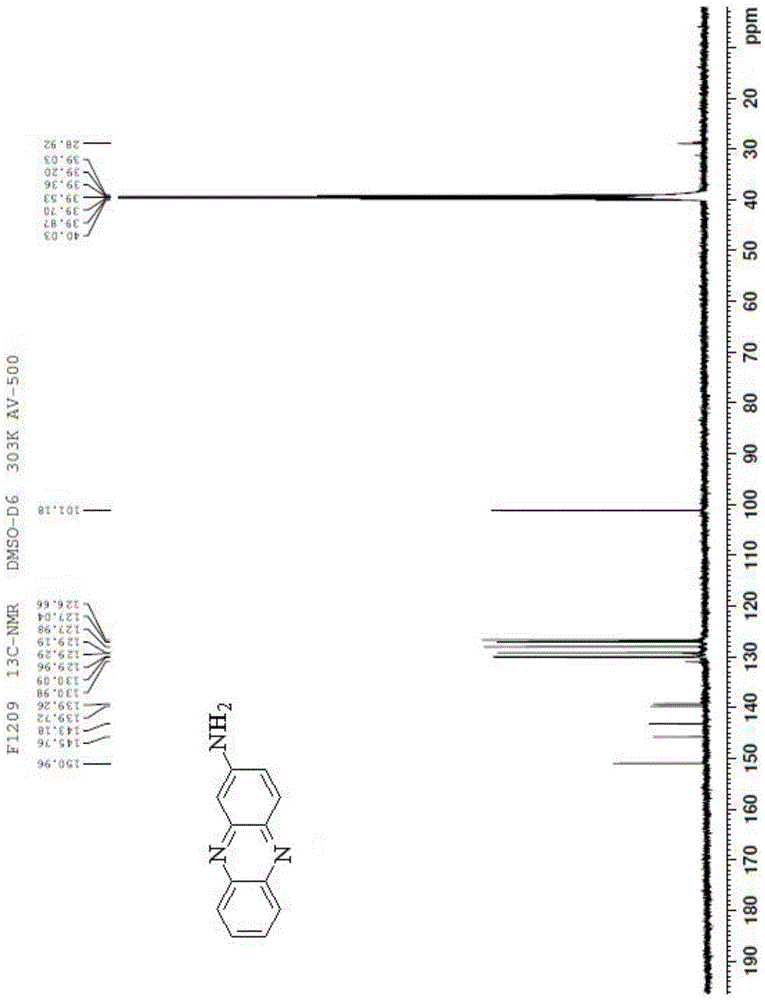
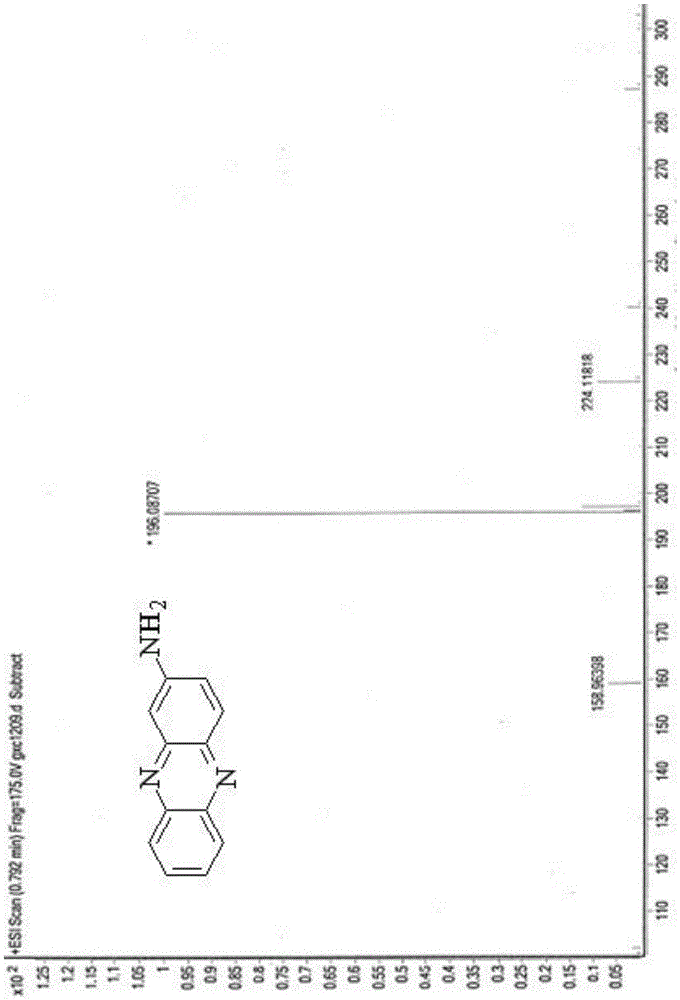
[0036] A kind of synthesis of the phenazine amides compound with antitumor activity, comprises the following steps:
[0037] Add 1.3g of aniline hydrochloride, 1.4g of o-nitroaniline and 4.2g of zinc chloride into the reaction flask, and stir the reaction at 160°C for 10min. After the reaction, cool the reactant to 25°C and pour Adding mass concentration is 25% sodium hydroxide solution 15ml to neutralize the acid generated by the reaction, remove the liquid obtained in the reaction flask by filtration, add 70ml ethanol to the obtained filter residue and reflux, collect the extract, and after reflux for 25min, pour the obtained filter residue Then add 70ml of ethanol to continue to reflux, collect the extract, and after reflux for 25min, add 70ml of ethanol to the obtained filter residue to continue to reflux, collect the extract, combine the three extracts together, remove the liquid in the extract, and get the crude product , mix the crude product with silica gel, pass through...
Embodiment 2
[0040] A kind of synthesis of the phenazine amides compound with antitumor activity, comprises the following steps:
[0041] Add 2g of aniline hydrochloride, 2.1g of o-nitroaniline and 4.5g of zinc chloride into the reaction flask, and stir the reaction at 180°C for 15 minutes. After the reaction, cool the reactant to 27°C, and add Mass concentration is the acid that 18ml of sodium hydroxide solution 18ml of 25% neutralizes reaction to generate, filter and remove the liquid that obtains in the reaction flask, add 75ml ethanol and reflux in the obtained filter residue, collect extract, after reflux 30min, pour into the obtained filter residue Add 75ml of ethanol again and continue to reflux, collect the extract, after reflux for 30min, add 75ml of ethanol again to the resulting filter residue and continue to reflux, collect the extract, combine the three extracts together, remove the liquid in the extract to obtain a crude product, Mix the crude product with silica gel, pass th...
Embodiment 3
[0044] A kind of synthesis of the phenazine amides compound with antitumor activity, comprises the following steps:
[0045] Add 2.6g of aniline hydrochloride, 2.8g of o-nitroaniline and 8.4g of zinc chloride into the reaction flask, and stir the reaction at 200°C for 20min. Adding mass concentration is 25% sodium hydroxide solution 20ml to neutralize the acid generated by the reaction, remove the liquid obtained in the reaction flask by filtration, add 80ml ethanol to the obtained filter residue and reflux, collect the extract, and after reflux for 35min, pour the obtained filter residue Add 80ml of ethanol to continue to reflux, collect the extract, and after reflux for 35min, add 80ml of ethanol to the obtained filter residue to continue to reflux, collect the extract, combine the three extracts together, remove the liquid in the extract, and obtain the crude product , mix the crude product with silica gel, pass through the column, use petroleum ether-ethyl acetate system, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com