Preparing method of ultraviolet absorber UV-329
A technology of UV-329 and absorbent, applied in the field of preparation of ultraviolet absorbent UV-329, can solve the problems of environmental pollution, high cost, low yield, etc., and achieve the effects of reducing the generation of impurities, low cost, and low three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
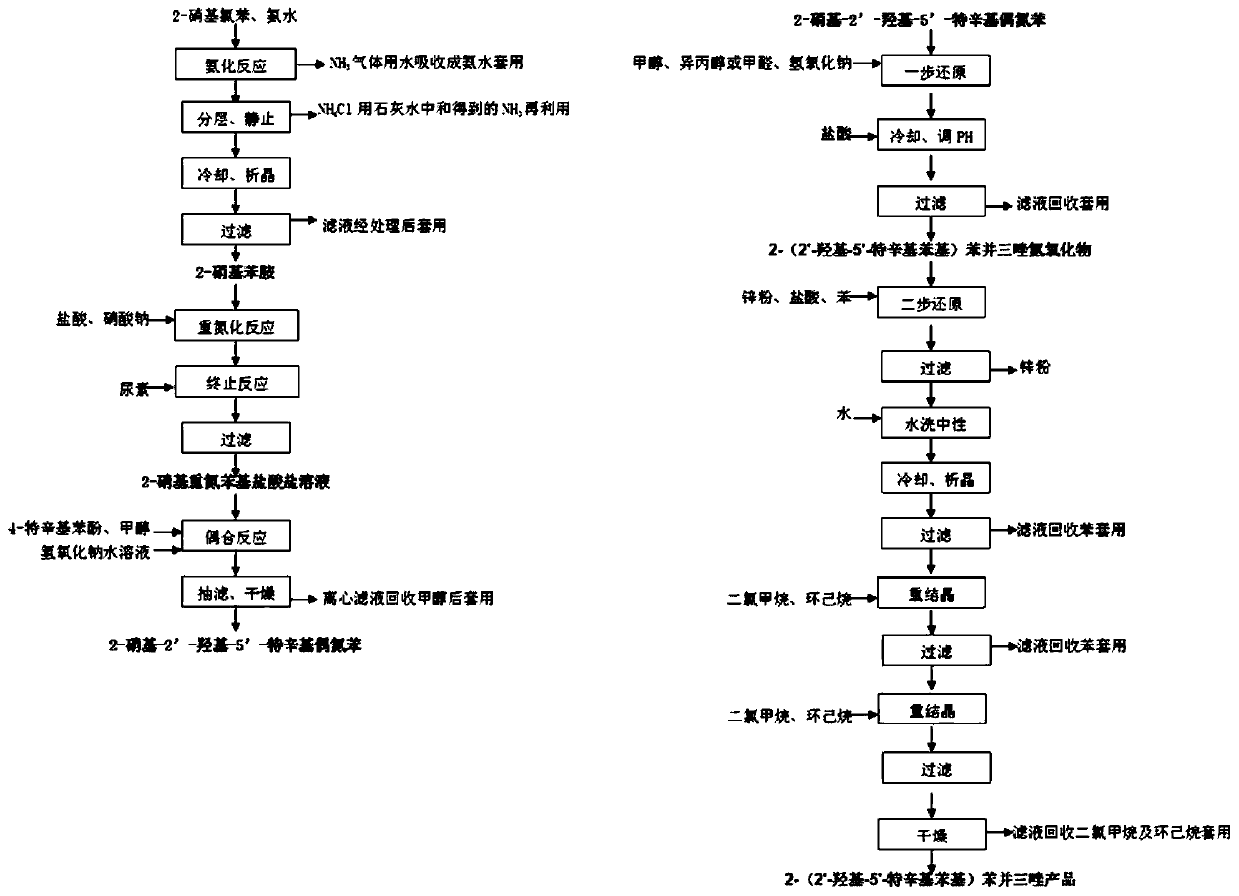
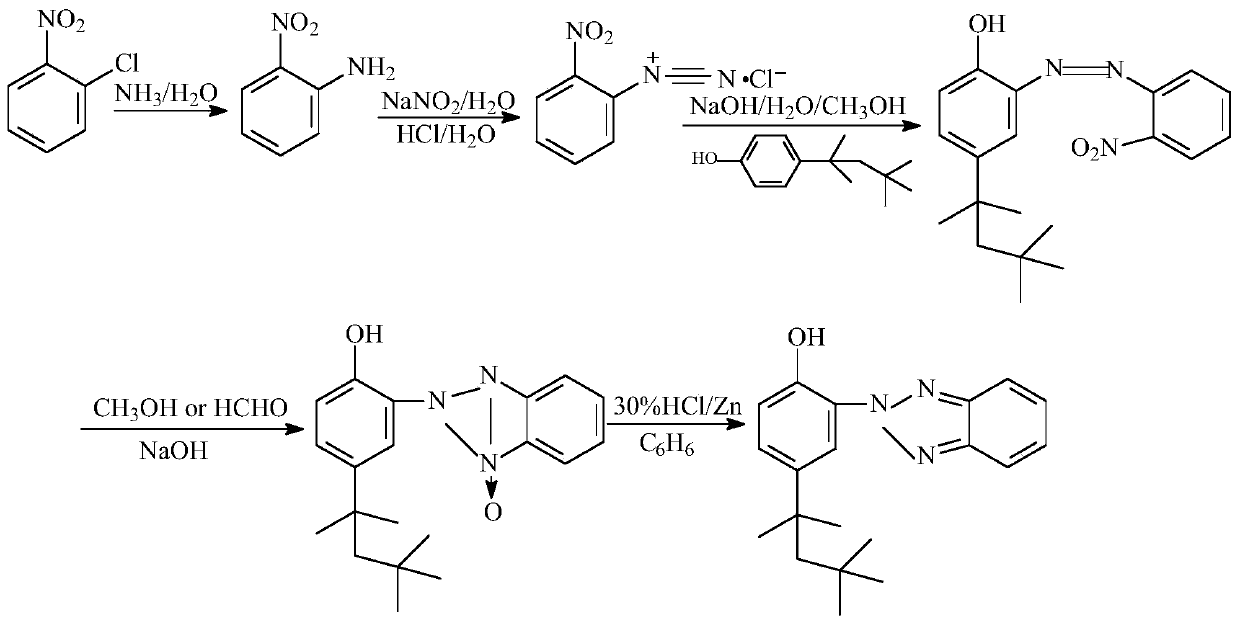
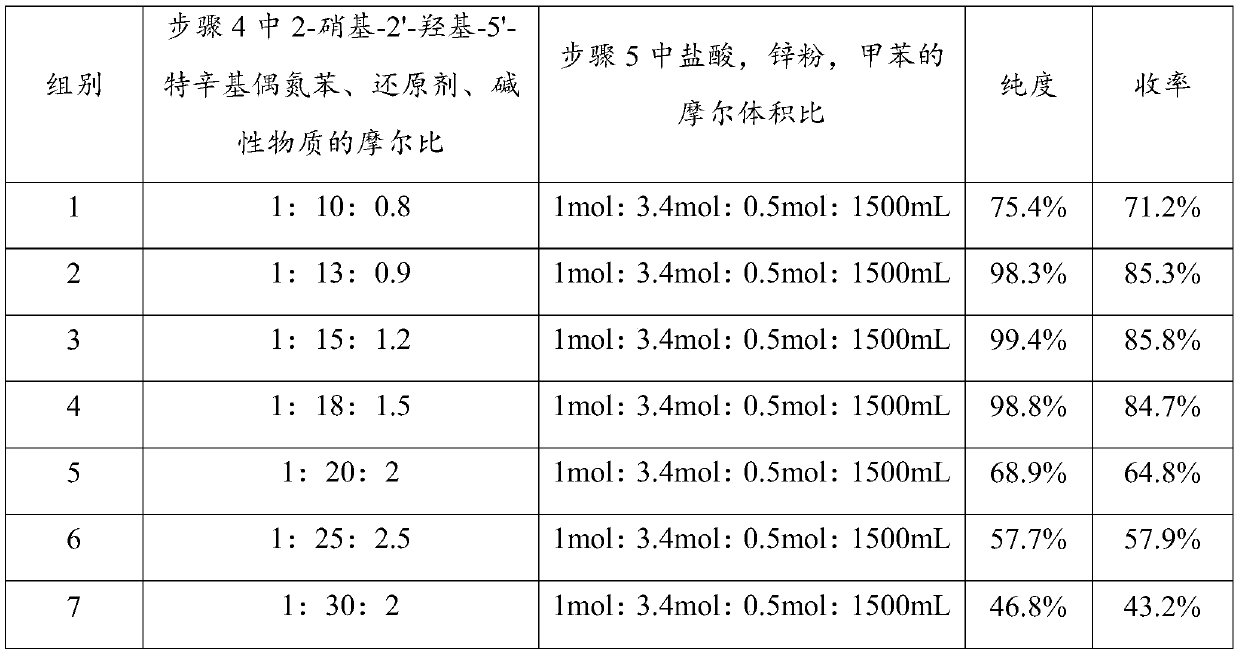
[0033] The present invention prepares 2-(2'-hydroxyl-5'-tertoctylphenyl)benzotriphenylene from o-nitrochlorobenzene through ammoniation, diazotization, coupling, one-step reduction, two-step reduction reaction and post-treatment process azole products.
[0034] Step 1, the preparation of 2-nitroaniline: put 1mol of 2-nitrochlorobenzene and 20mol of 20% ammonia water into the pipeline type ammoniation reactor and perform the ammoniation reaction at 150~220°C and 12~20MPa for 30~ After 50 minutes, the residual ammonia in the system was sprayed to circulate and absorb the low-concentration ammonia water produced, and after adding part of the liquid ammonia, it continued to be used for the ammonification reaction. neutralize and recover ammonia in water), and obtain 2-nitroaniline product through cooling and crystallization, with a yield of ≥98.6% and a purity of ≥99.6%.
[0035] Step 2. Preparation of 2-nitrodiazophenyl hydrochloride: Add 1mol of 2-nitroaniline, 3-4mol of 31% hy...
Embodiment 2
[0040] The present invention prepares 2-(2'-hydroxyl-5'-tertoctylphenyl)benzotriphenylene from o-nitrochlorobenzene through ammoniation, diazotization, coupling, one-step reduction, two-step reduction reaction and post-treatment process azole products.
[0041] Step 1. Preparation of 2-nitroaniline: put 1mol of 2-nitrochlorobenzene and 22mol of 10% ammonia water into the pipeline ammoniation reactor, and perform ammoniation reaction at 150~220°C and 12~20MPa for 30~ After 50 minutes, the residual ammonia in the system was sprayed to circulate and absorb the low-concentration ammonia water produced, and after adding part of the liquid ammonia, it continued to be used for the ammonification reaction. neutralize and recover ammonia in water), and obtain 2-nitroaniline product through cooling and crystallization, with a yield of ≥98.3% and a purity of ≥99.2%.
[0042] Step 2. Preparation of 2-nitrodiazophenyl hydrochloride: Add 1 mol of 2-nitroaniline, 3 mol of 5% hydrochloric ac...
Embodiment 3
[0047] The present invention prepares 2-(2'-hydroxyl-5'-tertoctylphenyl)benzotriphenylene from o-nitrochlorobenzene through ammoniation, diazotization, coupling, one-step reduction, two-step reduction reaction and post-treatment process azole products.
[0048] Step 1. Preparation of 2-nitroaniline: put 1mol of 2-nitrochlorobenzene and 12mol of 25% ammonia water into a pipeline ammoniation reactor and perform ammoniation reaction at 150-220°C and 12-20MPa for 30- After 50 minutes, the residual ammonia in the system was sprayed to circulate and absorb the low-concentration ammonia water produced, and after adding part of the liquid ammonia, it continued to be used for the ammonification reaction. neutralize and recover ammonia in water), and obtain 2-nitroaniline product through cooling and crystallization, with a yield of ≥98.2% and a purity of ≥99.5%.
[0049] Step 2, preparation of 2-nitrodiazophenyl hydrochloride: add 1mol of 2-nitroaniline, 4mol of 35% hydrochloric acid a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com