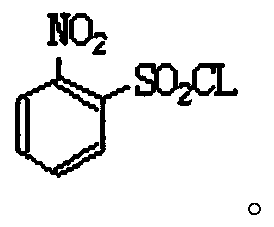
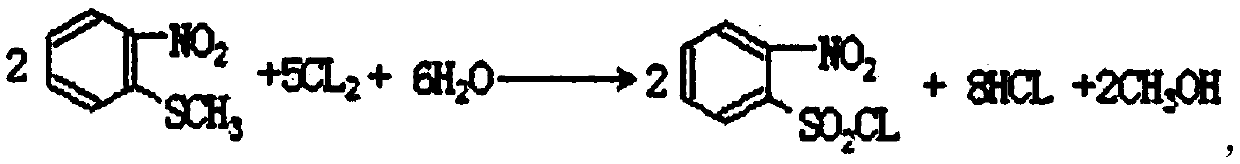Industrial production method of o-nitrobenzenesulfonyl chloride
A technology of nitrobenzenesulfonyl chloride and production method, applied in chemical instruments and methods, sulfonic acid preparation, organic chemistry and other directions, can solve problems such as low chlorine flow efficiency, reduced solvent toxicity, shortened process route, etc., and achieves the solution of reactants Product compatibility, reducing exhaust emissions, and improving reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
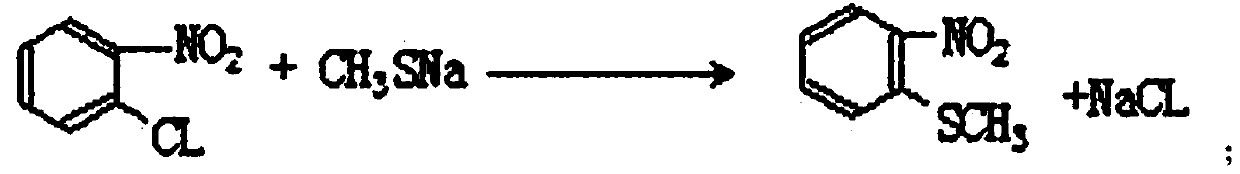
[0041] The synthetic method of o-nitrobenzenesulfonyl chloride comprises the steps:
[0042] (1) Add melted o-nitrochlorobenzene in the etherification reaction kettle, and then gradually add liquid sodium methylmercaptide dropwise to carry out etherification reaction. The etherification reaction conditions are: etherification when adding sodium methylmercaptide dropwise The temperature in the reaction kettle is 40-70°C, the dropwise addition time is 3-5 hours, and after the dropwise addition is completed, the temperature is kept at 75-80°C, the holding time is 2-3 hours, and the rotation speed is 60-100 rpm.
[0043] (2) Filter after the etherification reaction, and then recrystallize the obtained filter cake in a hydrophilic alcohol solvent, which is methanol, ethanol, propanol or ethylene glycol, and divide it five times Carrying out centrifugal separation and drying until there is no liquid in the bottom layer, and drying to obtain a wet product of o-nitroanisole sulfide; m...
Embodiment 1
[0049] The synthetic method of o-nitroanisole sulfide comprises the steps:
[0050] (1) Add 79kg (1.8 parts by weight) of fused o-nitrochlorobenzene in a 500L etherification reactor, then inject 193kg (4.4 parts by weight) of liquid sodium methyl mercaptide to carry out etherification reaction. The reaction conditions are as follows: the sodium methylthiolate solution is added dropwise at a temperature of 40°C for 5 hours during etherification, and after the dropwise addition, it is raised to 75°C and kept for 3 hours at a speed of 60 rpm. The total etherification reaction is about 8 hours. Hour;
[0051] (2) Filter after the etherification reaction, then recrystallize the obtained filter cake in ethanol for 1 hour, and carry out centrifugation and drying in five times until the bottom layer has no liquid, and after drying, o-Nitroanisyl sulfide wet product; move the wet product into a vacuum desiccator to obtain a dry product of o-nitroanisole sulfide, with a yield of 96%; ...
Embodiment 2
[0053] The synthetic method of o-nitrobenzenesulfonyl chloride comprises the steps:
[0054] (1) Add 79kg (1.8 parts by weight) of fused o-nitrochlorobenzene in a 500L etherification reactor, then inject 193kg (4.4 parts by weight) of liquid sodium methyl mercaptide to carry out etherification reaction. The reaction conditions are: during etherification, the sodium methylthiolate solution is added dropwise at a temperature of 70°C for 5 hours. After the dropwise addition, the temperature is raised to 80°C for 3 hours, and the etherification reaction is about 8 hours at a speed of 60 rpm. ;
[0055] (2) Filter after the etherification reaction, then recrystallize the obtained filter cake in ethanol for 4 hours, and carry out centrifugation and drying in five times until there is no liquid in the bottom layer, and after drying, the wet product of o-nitrophenyl sulfide is obtained ; Move the wet product into a vacuum desiccator to obtain a dry product of o-nitrophenyl sulfide, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| turbidity | aaaaa | aaaaa |
| turbidity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com