Method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene
A technology for p-nitroanisole and o-nitroanisole is applied in the field of preparing o-nitroanisole and p-nitroanisole, and can solve the problem of intractable waste residue, poor operation safety performance and harsh operating conditions and other problems to achieve the effect of reducing production costs and saving energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0019] The present invention will be further described with reference to the following examples, but it should be understood that these examples are for illustrative purposes only and should not be construed as limitations on the implementation of the present invention.
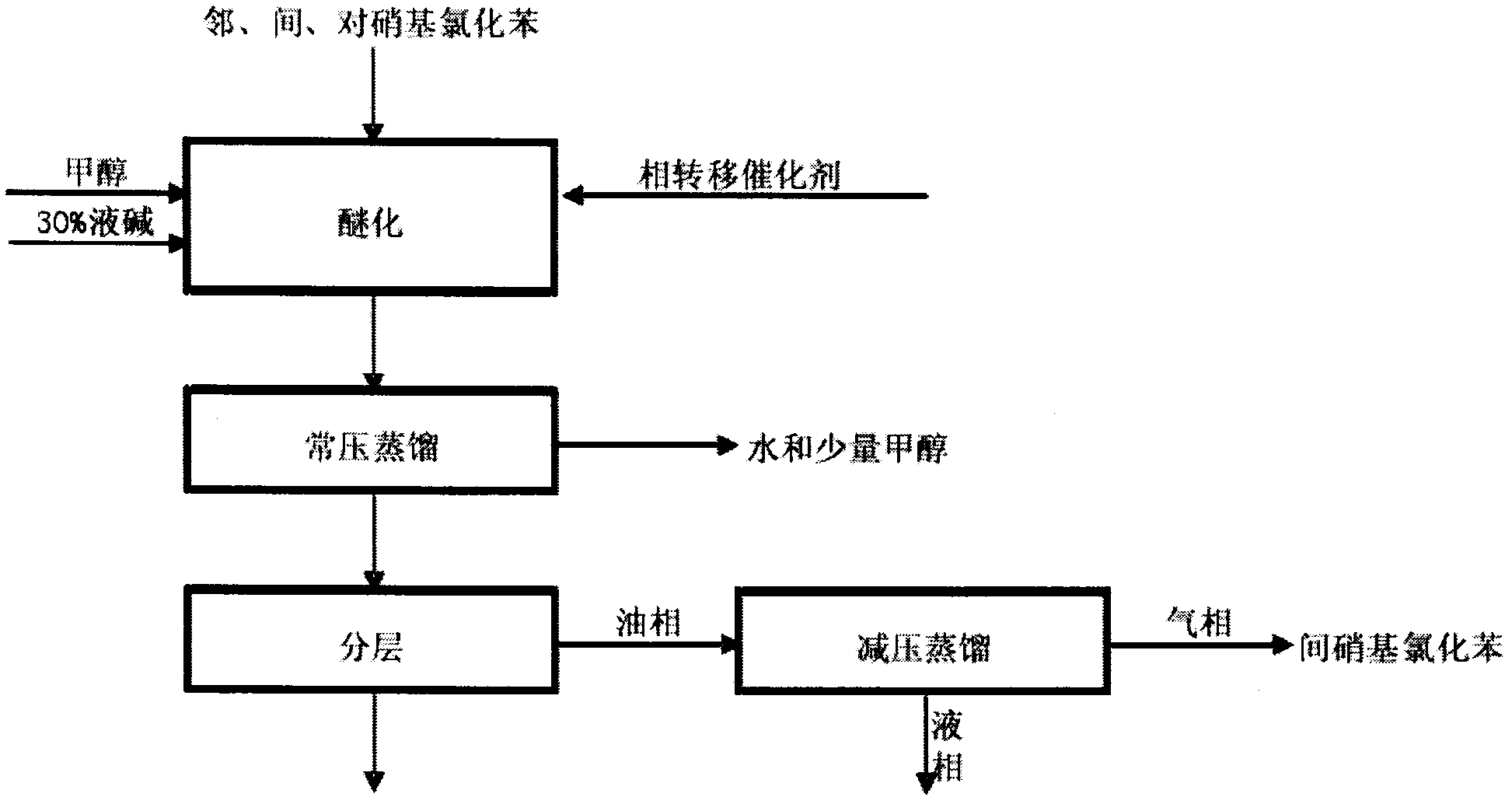
[0020] The present invention uses the products obtained by the nitration of chlorobenzene, ortho-nitrochlorobenzene, m-nitrochlorobenzene and p-nitrochlorobenzene, and uses this mixed nitrochlorobenzene as a raw material. In the methanol system, add a certain The amount of 30% lye and phase-transfer catalyst react to produce o-nitroanisole and p-nitroanisole products, and m-nitrochlorobenzene does not participate in these reactions.
[0021] The process is as follows:
[0022] (1) Etherification
[0023] The nitrochlorobenzene mixture is dissolved in methanol system, 30% liquid caustic soda and phase transfer catalyst are added, and the reaction is carried out for 6 hours.
[0024] (2) Atmospheric distillat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com