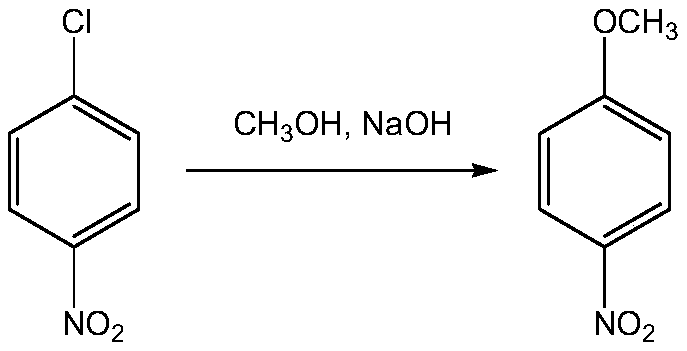
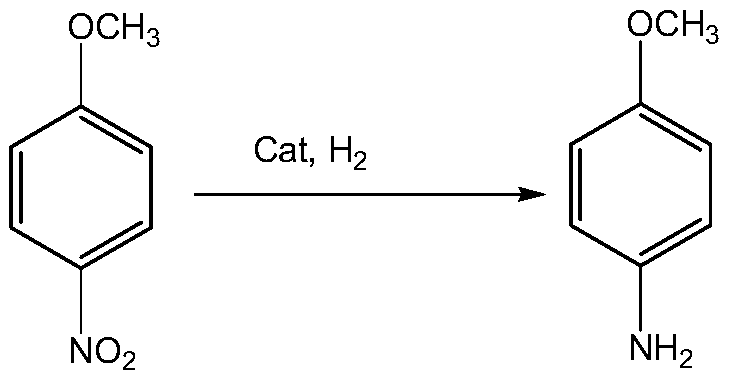
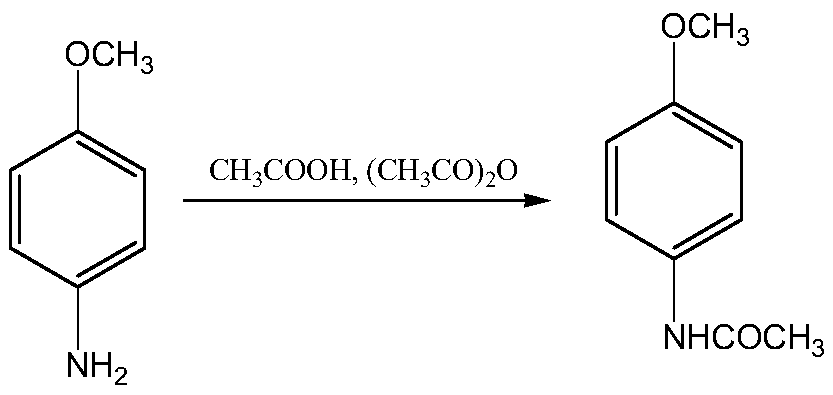
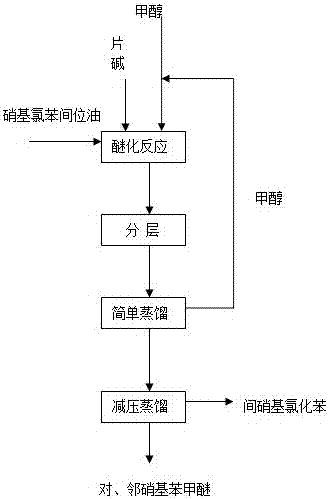
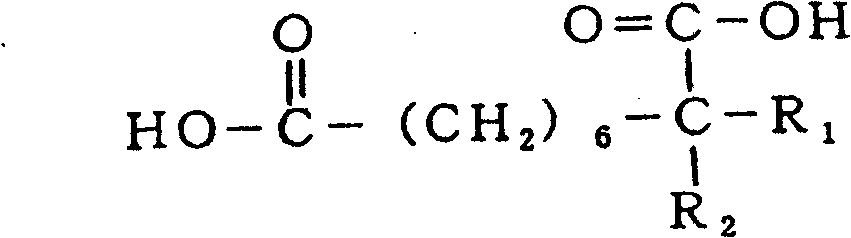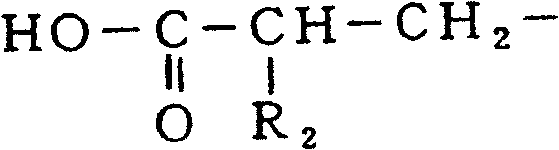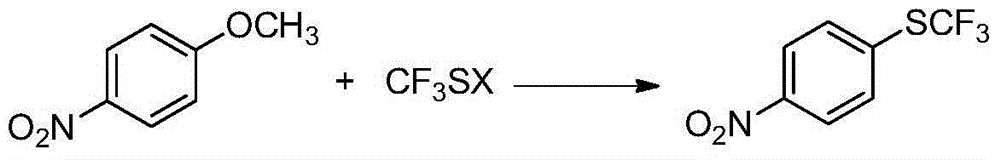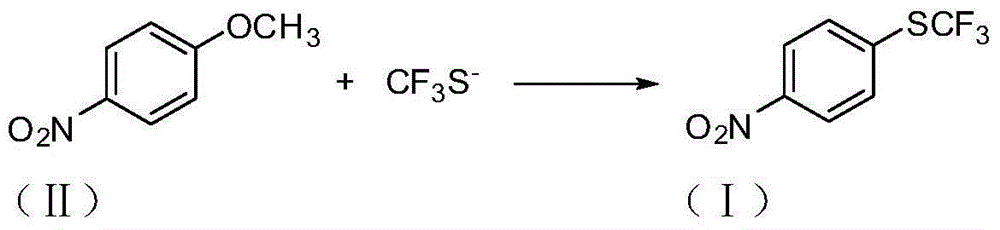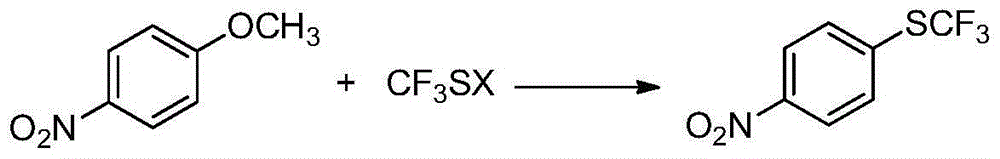Patents
Literature
42 results about "P-nitroanisole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
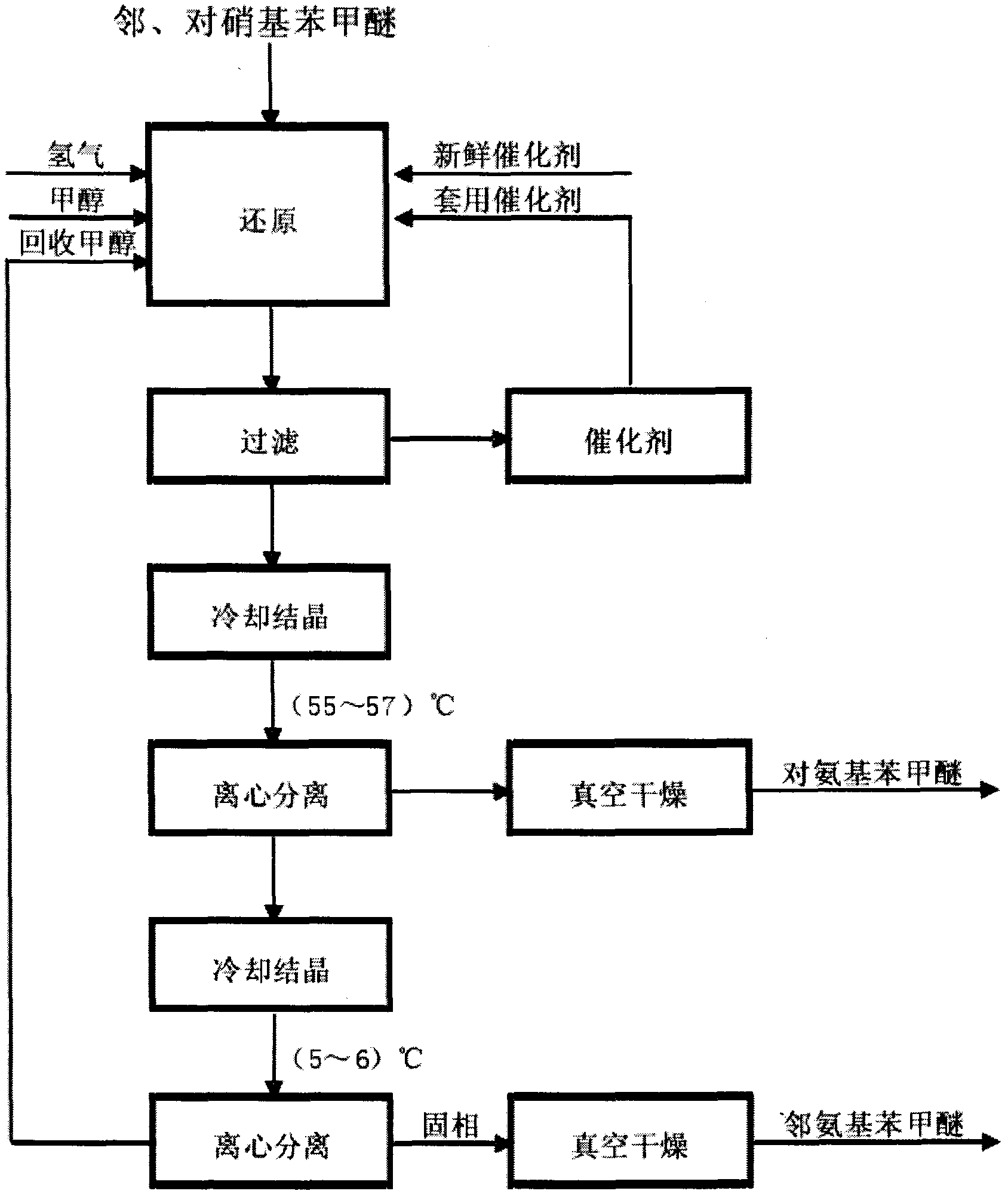
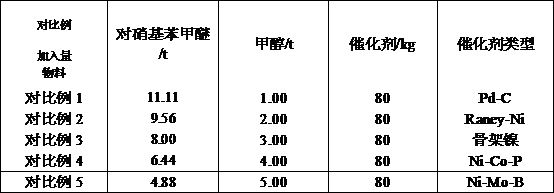
Preparation method for p-anisidine
ActiveCN105272863AHigh selectivityMild reaction conditionsOrganic compound preparationChemical recyclingP-nitroanisoleAlloy catalyst
The invention relates to a preparation method for p-anisidine. In the presence of a ternary amorphous alloy catalyst being Ni-Mo-B or Ni-Co-P, liquid-phase p-nitroanisole is subjected to hydrogenation through intermittent hydrogenation, and therefore p-anisidine with a selectivity of more than 99.7% can be obtained. In the preparation method, reaction conditions are mild, the solvent and the catalyst can be recycled, and the preparation method is liable to industrial production.
Owner:NINGXIA ZHONGSHENG NEW TECH CO LTD
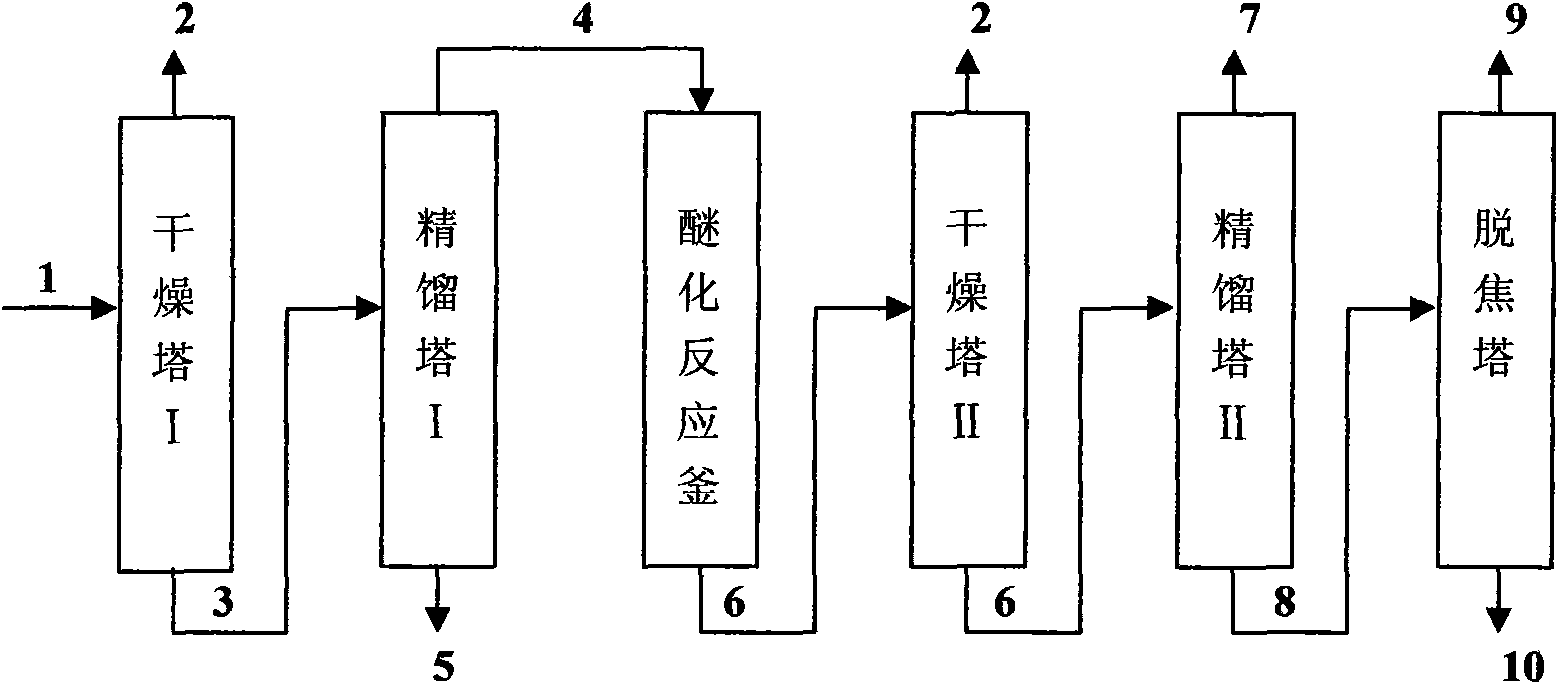
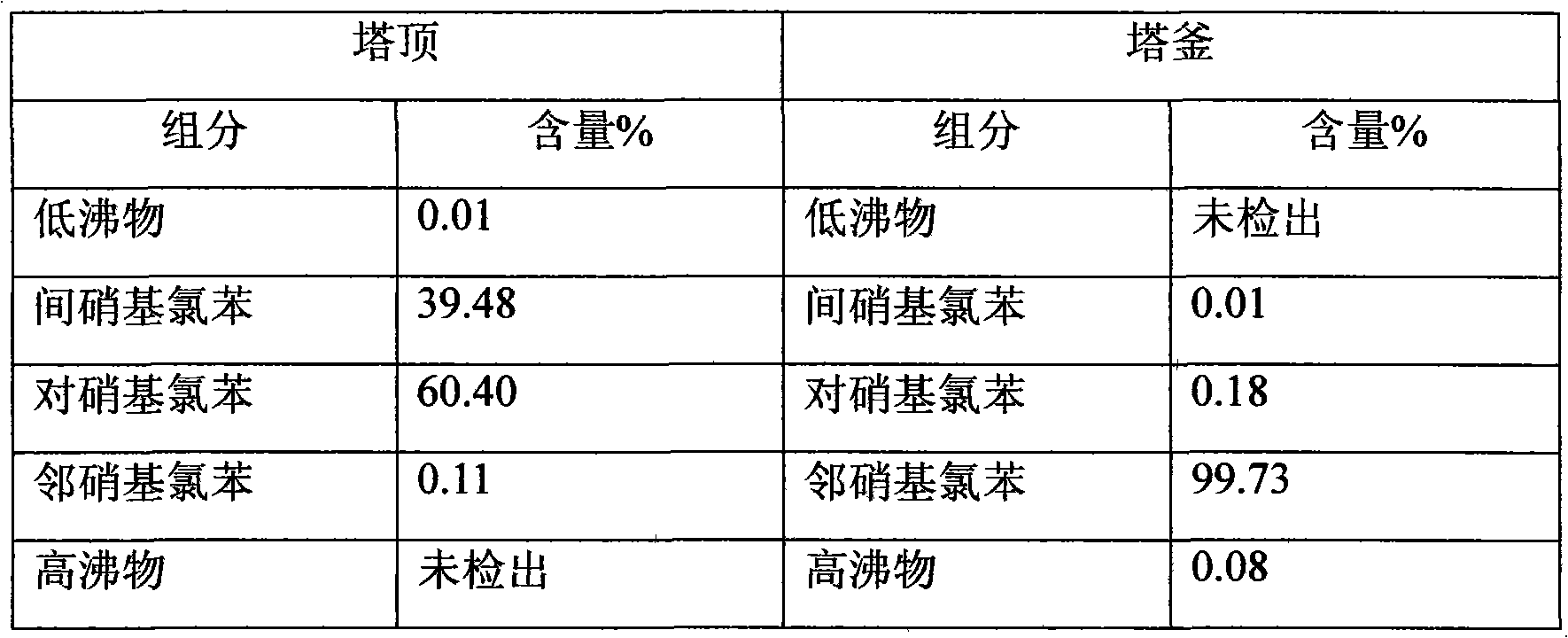
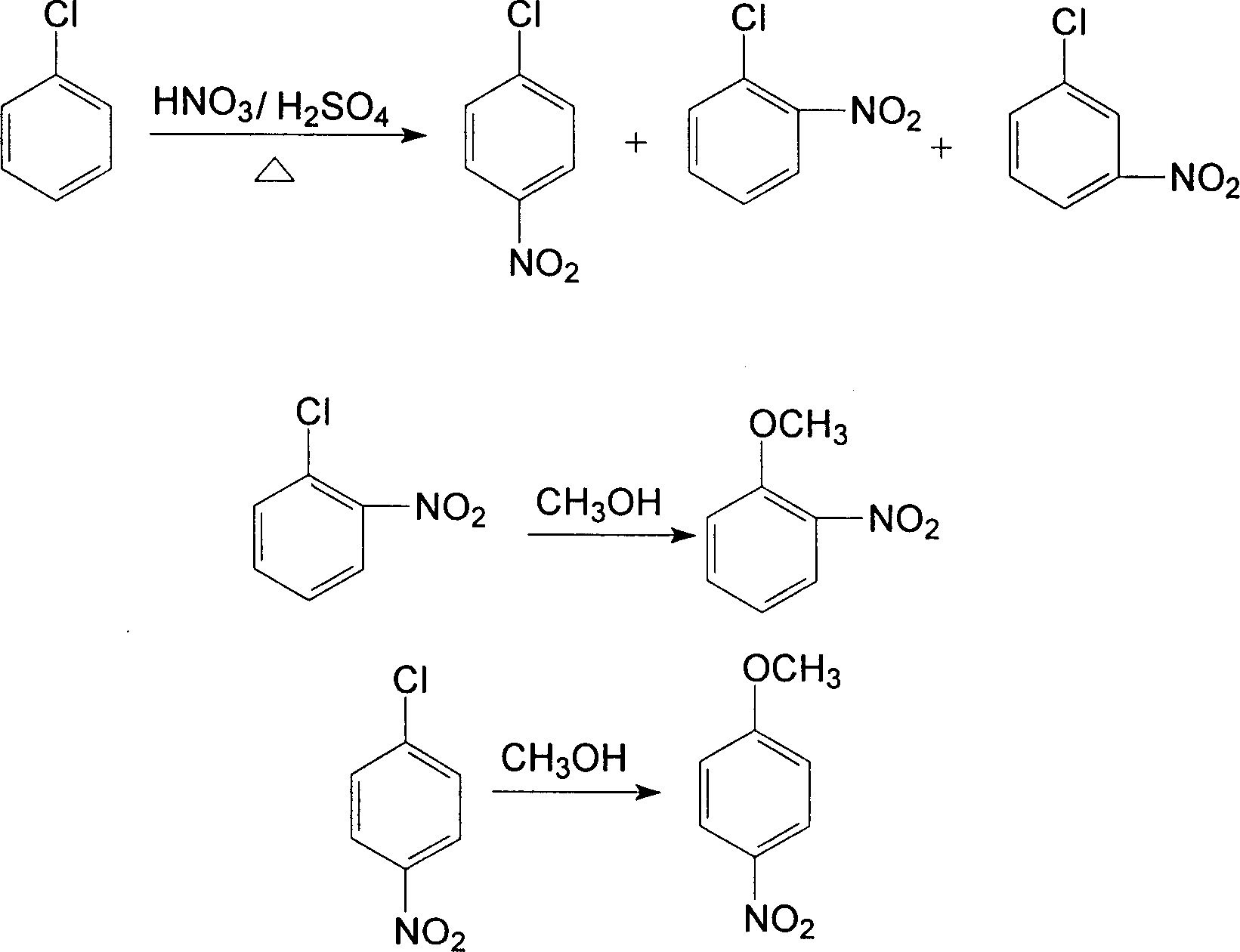
Method for preparing m-nitrochlorobenzene, o-nitrochlorobenzene and p-nitrochlorobenzene by using nitrochlorobenzene meta-position oil
InactiveCN101935281AAvoid pollutionEasy to separateOrganic chemistryOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
The invention relates to a method for preparing m-nitrochlorobenzene, o-nitrochlorobenzene and p-nitrochlorobenzene by using nitrochlorobenzene meta-position oil. The method comprises the following steps of: separating the nitrochlorobenzene meta-position oil containing 20 to 45 percent of m-nitrochlorobenzene and produced in the conventional nitrochlorobenzene production process into an m-nitrochlorobenzene and p-nitrochlorobenzene mixture and an o-nitrochlorobenzene product by a rectification method, further converting the m-nitrochlorobenzene and p-nitrochlorobenzene mixture into an m-nitrochlorobenzene and p-nitroanisole mixture by methoxylation reaction, and performing rectification, separation and purification on the mixture to obtain m-nitrochlorobenzene and p-nitroanisole products. The method has the advantages of low energy consumption, short production period and capability of solving the atmospheric pollution problem of a burning treatment method for the nitrochlorobenzene meta-position oil.
Owner:邵阳市申强化工有限责任公司
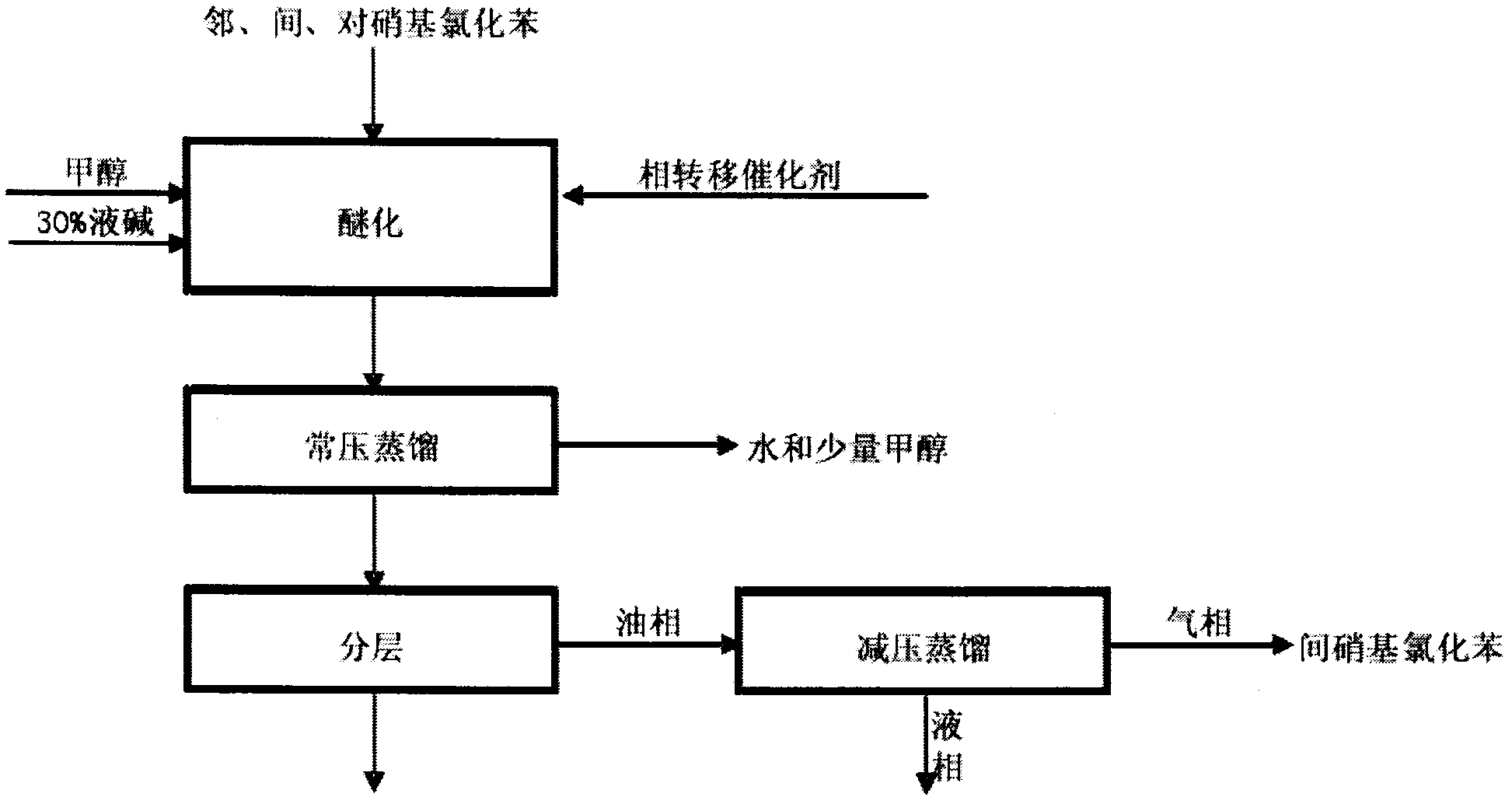
Method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene
InactiveCN103073431AAvoid costly separationsSave energyOrganic chemistryOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
The invention relates to a method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene. According to the invention, mixed nitrochlorobenzene consisting of a product obtained after nitration of chlorobenzene, o-nitrochlorobenzene, m-nitrochlorobenzene and p-nitrochlorobenzene is used as a raw material, and in a methanol system, 30% of alkali lye and a phase-transfer catalyst are added for a reaction to prepare products of o-nitroanisole and p-nitroanisole, wherein m-nitrochlorobenzene does not participate in the reaction. The method provided by the invention overcomes the problems of great investment for rectifying tower equipment, great energy consumption in rectification, high cost, long process flow, harsh operation conditions, poor operational safety performances and generation of waste residues hardly to treat in rectification-crystallization separation of mixed nitrochlorobenzene in conventional production processes for o-nitroanisole and p-nitroanisole and overcomes the technical problems of generation of considerable alkaline waste water containing sodium hyposulfite and production of a poor-quality product in a sodium sulfide reduction process.
Owner:CHANGZHOU JIASEN CHEM
Method for preparing nitroanisole from m-nitrochlorobenzene oil
ActiveCN104557557AShort reaction timeThe reaction cycle is shortenedOrganic chemistryOrganic compound preparationChlorobenzeneP-nitroanisole
The invention aims to provide a nitroanisole production method with low cost, low energy consumption, short production cycle and few three wastes (waste gas, wastewater and industrial residue), namely a method for preparing m-nitrochlorobenzene, p-nitroanisole and o-nitroanisole from m-nitrochlorobenzene oil under an anhydrous system by a high pressure method. To achieve the above purpose, the technical scheme of the invention is as follows: m-nitrochlorobenzene oil is added into an autoclave, and methanol and sodium hydroxide are respectively added, wherein the mole ratio of sodium hydroxide to m-nitrochlorobenzene oil is 0.01-2.00:1, the mole ratio of methanol to m-nitrochlorobenzene oil is 1-20:1, reaction temperature is 10-200 DEG C, reaction time is 1-20 h, and pressure is 0.1-4.0 MPa; and products obtained after the reaction undergo gas chromatography, and content changes with the composition change of the reaction raw material m-nitrochlorobenzene oil. In comparison with traditional technologies, the method provided by the invention has advantages of low production cost, short process, low energy consumption and little pollution.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing p-anisidine through catalytic hydrogenation of p-nitroanisole
ActiveCN108047064AHigh activityHigh yieldMolecular sieve catalystsOrganic compound preparationP-nitroanisoleHydrogen pressure
The invention discloses a method for preparing p-anisidine through catalytic hydrogenation of p-nitroanisole. According to the method, p-nitroanisole is used as a reaction raw material and subjected to a reaction in an enclosed reactor with a mechanical stirring function under the conditions of usage of a composite catalyst, no usage of a solvent, a reaction temperature of 80 to 150 DEG C and hydrogen pressure of 0.3 to 1 MPa so as to obtain p-anisidine, wherein the composite catalyst is composed of active components metal M and a carrier; the active components metal M are composed of two or more selected from a group consisting of Cu, Ni, Fe, Zn, Co, Cr and Mo, and the content of the active components metal M accounts for 0.3 to 25 wt% of the mass of the composite catalyst; and the carrier is prepared by compounding a carbon-containing organic matter and an inorganic oxide, a mass ratio of the carbon-containing organic matter to the inorganic oxide is 1: 50 to 1: 10, and the inorganicoxide is any one selected from a group consisting of SiO2, Al2O3, MgO, TiO2, CeO2 and a 4A molecular sieve. The composite catalyst used in the invention has high catalytic activity, stable performance and high selectivity and conversion rate.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing p-anisidine through catalytic hydrogenation of p-nitroanisole
InactiveCN106892828AReduce consumptionAvoid generatingOrganic compound preparationAmino-hyroxy compound preparationNickel catalystP-nitroanisole
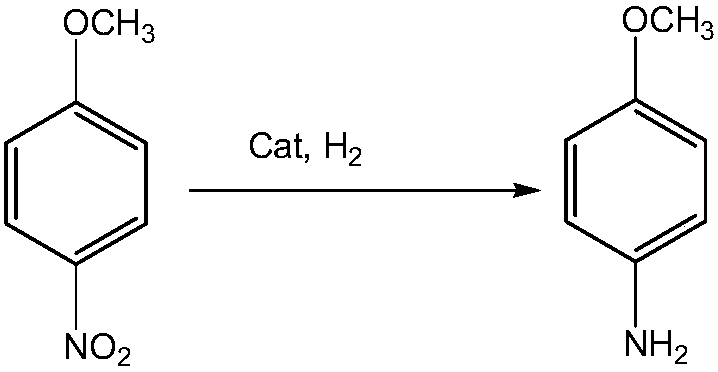
The invention provides a method for preparing p-aminoanisole by hydrogenating p-nitroanisole. In the presence of KT-02 supported nickel catalyst, the reduction reaction of p-nitroanisole can be started under relatively mild conditions to generate p-aminoanisole. Compared with the existing techniques such as nitrobenzene method, sodium sulfide or iron reduction method, the present invention has the advantages of mild reaction conditions, fast reaction speed, clean reaction process, easy separation of products and the like.
Owner:甘肃中科药源生物工程股份有限公司
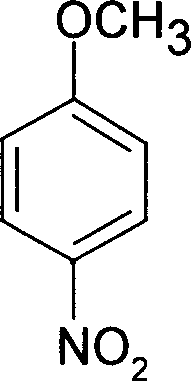
Catalysis synthesis method for nitrobenzene ether catalysis synthesis method for paranitroanisole
InactiveCN101314571AGreen synthesisEfficient synthesisOrganic chemistryOrganic compound preparationP-nitroanisoleAlkaline earth metal
The invention relates to a method for synthesizing paranitroanisole. The raw materials used by the method are green chemicals dimethyl carbonate (DMC) and p-nitrophenol, and the catalyst is NaY molecular sieve, activated aluminum oxide or activated carbon which is impregnated by alkali metals or alkaline-earth metal compounds. Good conversion rate of the raw materials and good selectivity of target products can be obtained by using the method provided by the invention under the reaction conditions that: the temperature is between 90 and 200 DEG C, and the reaction time is between 1 and 5 hours; the mol ratio of the p-nitrophenol to the dimethyl carbonate of the raw materials is between 1 to 5 and 1 to 15, and the amount of the catalyst is 2 to 20 percent of the mass of the raw materials, wherein, the conversion rate of the p-nitrophenol can reach over 64 percent, and the selectivity of the paranitroanisole can reach 100 percent. The method takes the DMC as a methylated reagent to actually realize a green synthetic route, takes solid alkali as the catalyst, and is superior to the prior synthetic method of homogeneous catalysis or phase-transfer catalysis; and the catalyst is simple to separate and can be reused.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
P-aminoanisole electrochemical synthesis method
InactiveCN101187032AMild reaction conditionsEasy to produceElectrolysis componentsElectrolytic organic productionSupporting electrolyteP-nitroanisole
Provided is an electrochemical synthetic method of aminoanisole, belonging to the technical field of electrochemistry. The method needs to be accomplished in a two-chamber electrolysis bath which is separated by employing a cation-exchange membrane, a copper sheet is taken as a negative electrode, a ruthenium net is taken as a positive electrode, a saturated calomel electrode is taken as a reference electrode, the negative electrode and the reference electrode are installed inside a cathode chamber of the electrolysis bath, and the positive electrode is installed inside an anode chamber of the electrolysis bath. Methanol is taken as solvent, sulphuric acid is taken as supporting electrolyte, paranitroanisole is taken as electrolytic reaction substrate, the solvent and the solution of supporting electrolyte are injected into the cathode chamber and the anode chamber, and the electrolytic reaction substrate is injected into the cathode chamber. The electrolyzation is performed within a condition of normal temperature and pressure and a condition that the negative electrode is added with a certain constant voltage relative to the reference electrode, and the constant voltage is between -0.4 to -0.6 V. After the electrolyzation is finished, the electrolyte is post-processed to obtain the product of paraphenetidine with the production ratio between 24.5-63.3%. The method has the advantages of simple requirement, mild reaction conditions, easy preparation of the electrodes, low price, small pollution in the process of reaction and the like, and is a greening production line.
Owner:EAST CHINA NORMAL UNIV
Microminiature rapid charging special-purpose aluminium electrolytic capacitor
InactiveCN106252083AGuaranteed capacityGuaranteed high withstand voltage performanceCapacitor housing/encapsulationP-nitroanisoleLightning strike
The invention provides a microminiature rapid charging special-purpose aluminium electrolytic capacitor which comprises a shell, a core bag and a rubber plug. The core bag is sealed in the shell through the rubber plug. The core bag contains electrolyte which comprises a solvent, an inorganic acid ammonium salt, an organic acid ammonium salt, and a hydrogen elimination agent. The inorganic acid ammonium salt is ammonium borate or ammonium pentaborate or the mixture of the ammonium borate or the ammonium pentaborate. The organic acid ammonium salt comprises one or more of ammonium sebacate, ammonium azelate, diammonium phthalate, and dodecyl ammonium carboxylate. The hydrogen elimination agent comprises one or more of a p-Nitrobenzyl alcohol, a p nitroanisole and p-nitrobenzoic acid. The conductivity of the electrolyte in the invention is between 2.4 ms / cm to 2.8ms / cm, the ESR value of a product can be effectively reduced, thus the ripple resistance and lightning strike resistance ability of the product are improved greatly, the flash fire voltage is above 490V, in a condition of high voltage, the breakdown of electrolytic paper caused by flash fire of the electrolyte does not appears, and thus the voltageproof performance of the product is ensured.
Owner:HUNAN AIHUA GRP
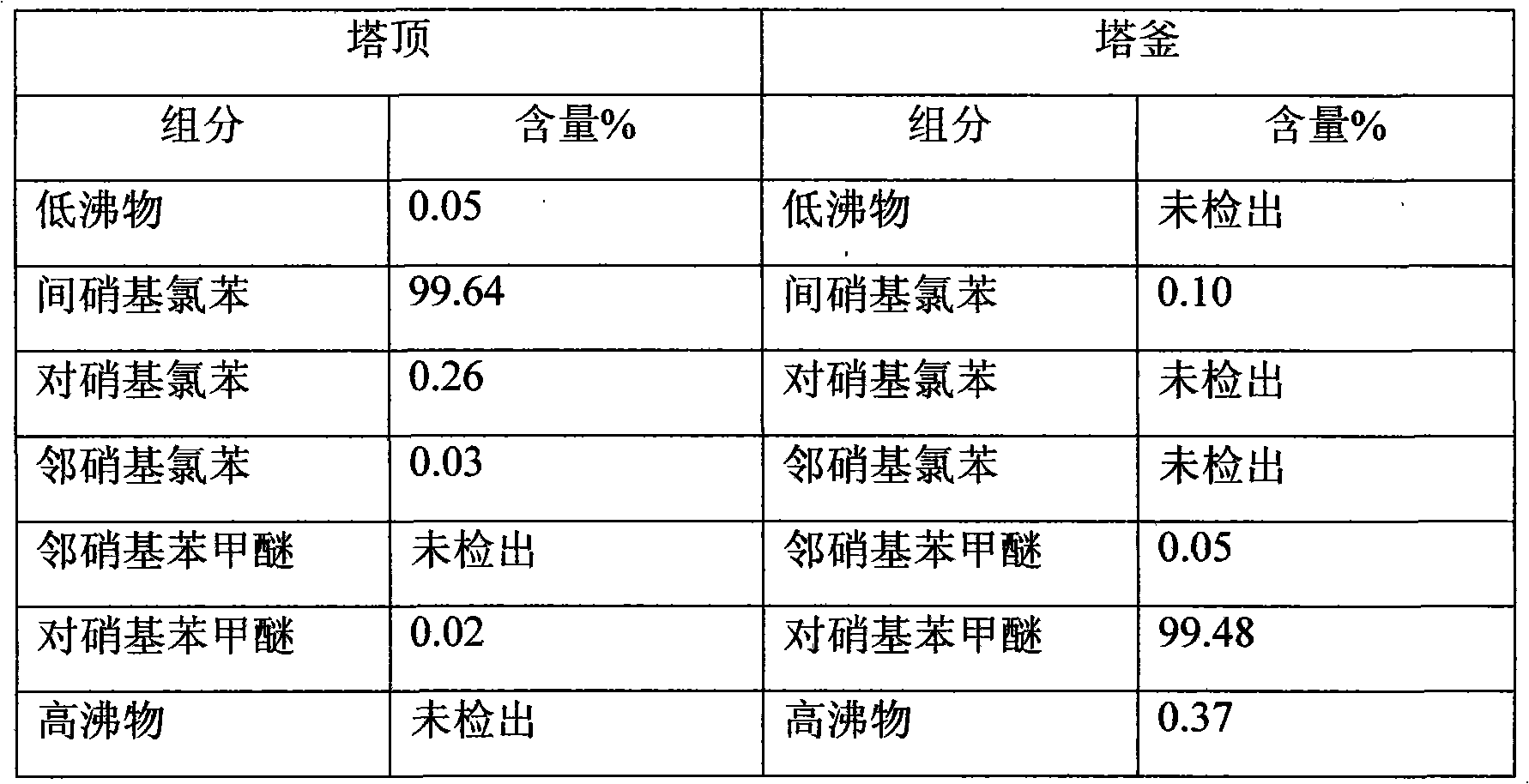
Clean production method of paranitroanisole
ActiveCN105399634AHigh yieldHigh purityOrganic chemistryOrganic compound preparationP-nitroanisoleDistillation
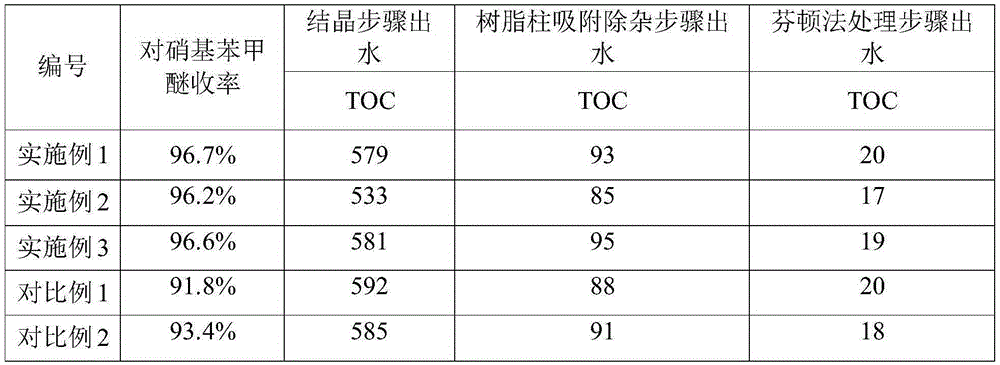
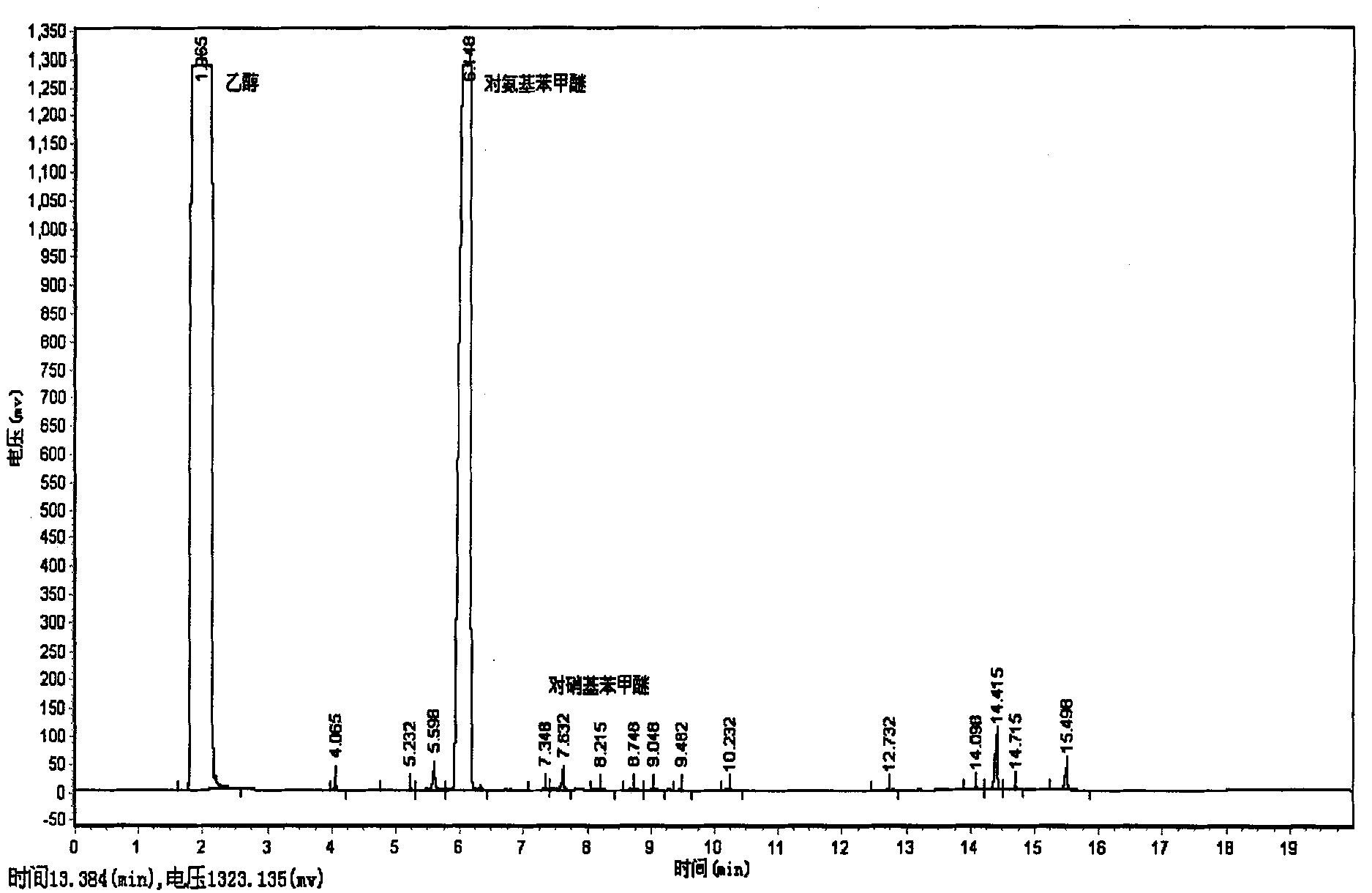
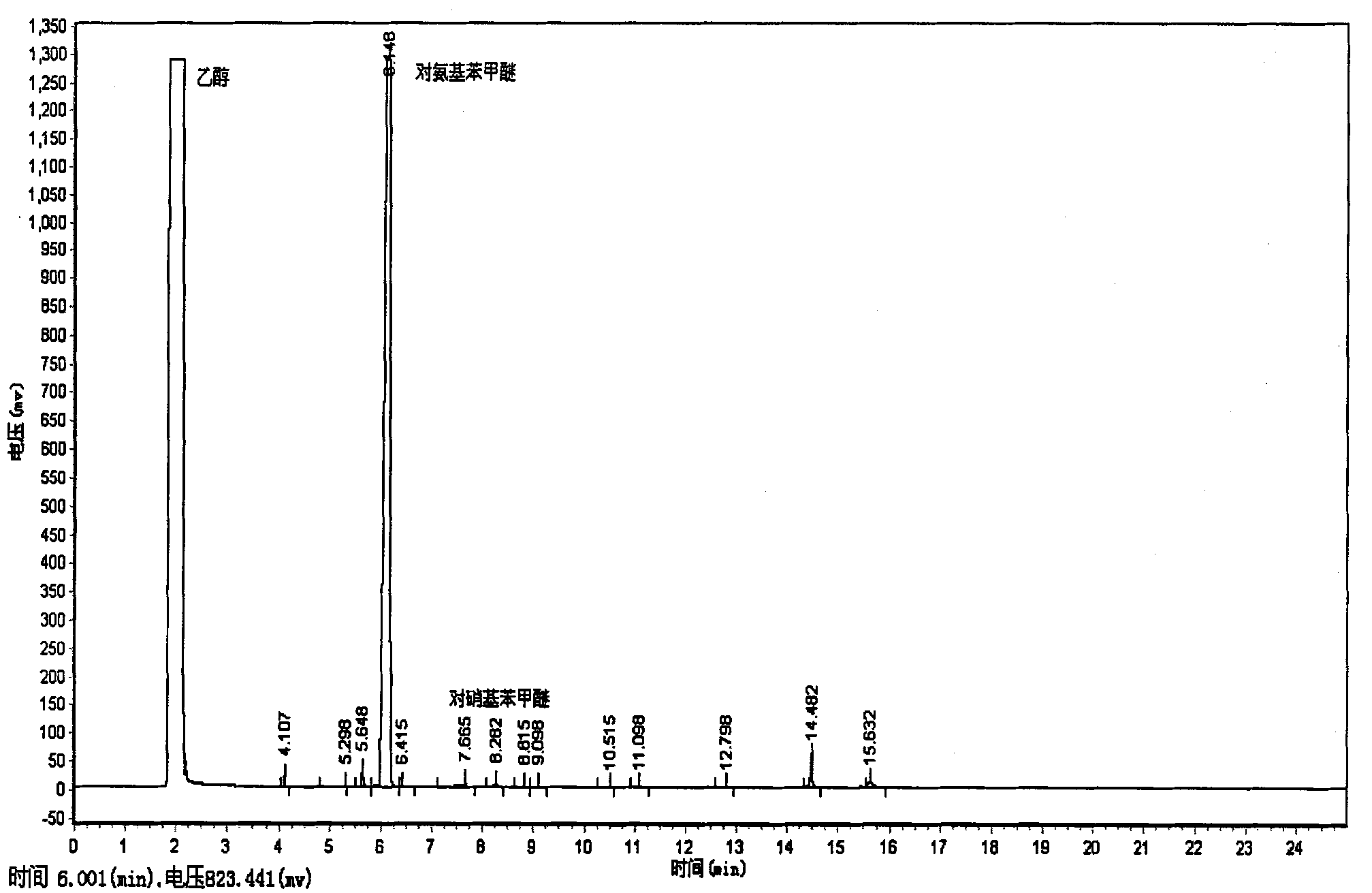
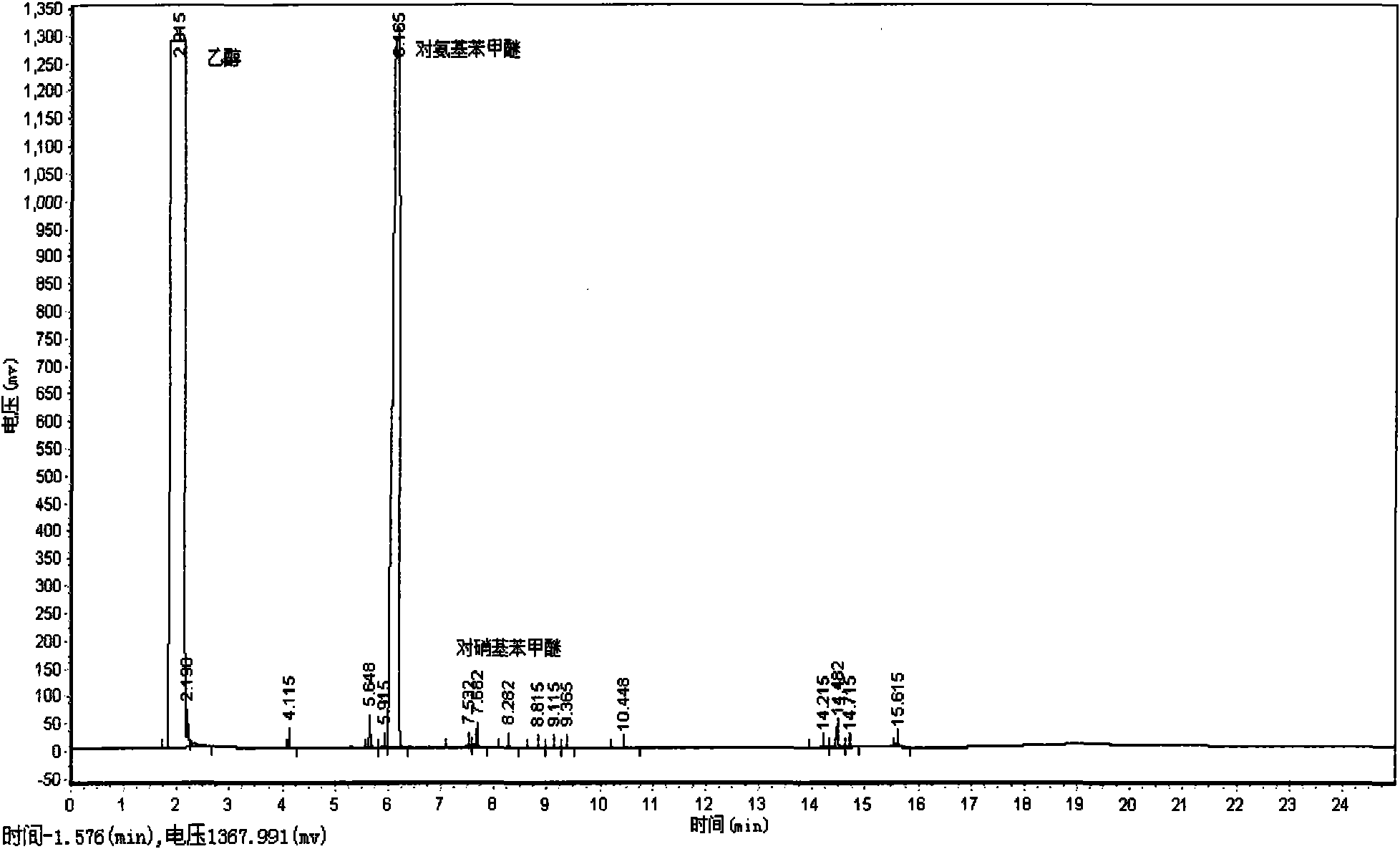
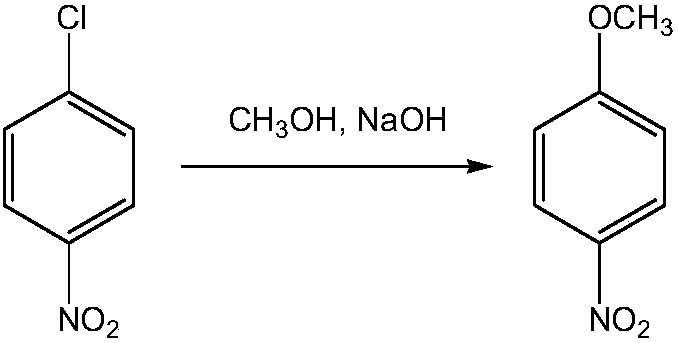
The invention provides a clean production method of paranitroanisole. The method comprises the step that sodium hydroxide, para-nitrochlorobenzene and methanol react to produce the paranitroanisole, and the reaction refers to that a sodium hydroxide methanol solution is added to a para-nitrochlorobenzene methanol solution in batches under gradient temperature rise. Besides, the clean production method further comprises the step of treatment of a reaction product mixture: the reaction product mixture is distilled, water is added to a reaction kettle after distillation for washing, standing and layering, and the paranitroanisole is obtained from an organic phase. With the adoption of the method, on the premises that side products are turned into wealth and TOC in wastewater is treated to be lower than 20, the yield of the paranitroanisole can be significantly increased and can be higher than 96% particularly.
Owner:ZHEJIANG RUNTU INST
Method for synthesizing para aminophenylmethylether by catalytic hydrogenation of paranitroanisole
InactiveCN101798272AEasy to separateImprove protectionOrganic compound preparationBulk chemical productionP-nitroanisoleHydrazine compound
The invention provides a method for synthesizing para aminophenylmethylether by catalytic hydrogenation paranitroanisole. In the method, the paranitroanisole is subjected to catalytic reduction reaction with hydrogen to generate the para aminophenylmethylether in the presence of a load type palladium catalyst in a supercritical carbon dioxide reaction medium under mild conditions. Compared with the traditional processes, such as a nitrobenzene method, a sodium sulfide or ferrum reduction method, a method for synthesizing the para aminophenylmethylether by reducing the paranitroanisole by activated carbon load type palladium catalyst hydrazine hydrate, and the like, the invention has the advantages of mild reaction condition, fast reaction, clean reaction process and easy product separation, achieving the product yield more than 99 percent, and the like; in addition, in the invention, reaction temperature is reduced from 75-125 DEG C in the nitrobenzene method to 50-80 DEG C, and reaction time is reduced from 130 minutes in the method for synthesizing the para aminophenylmethylether by reducing the paranitroanisole by the activated carbon load type palladium catalyst hydrazine hydrate to 10-30 minutes without adding any organic solvent and additive or generating any side products in the reaction process.
Owner:CHANGCHUN UNIV OF TECH
Method of preparing o-anisidine and p-anisidine through hydrogenation reduction of mixture of o-nitroanisole and p-nitroanisole
InactiveCN103073436ASimple processImprove conversion rateOrganic compound preparationChemical recyclingP-nitroanisoleO-Nitroanisole
The invention relates to a method of preparing o-anisidine and p-anisidine through hydrogenation reduction of a mixture of o-nitroanisole and p-nitroanisole. According to the method, o-nitroanisole and p-nitroanisole are used as raw materials, and o-anisidine and p-anisidine are prepared in a methanol system through hydrogenation reduction with Pt / C as a catalyst. With the method provided by the invention, the disadvantages of high energy consumption and high cost in conventional production process of o-anisidine and p-anisidine are overcome, and the technical problems of severe environmental pollution and loss of materials caused by considerable process waste water generated in the process of pretreatment in conventional production process are overcome; the method provided by the invention has the advantages of easiness, a high conversion rate, clean, green and environment-friendly process and a small amount of pollution by three wastes.
Owner:CHANGZHOU JIASEN CHEM
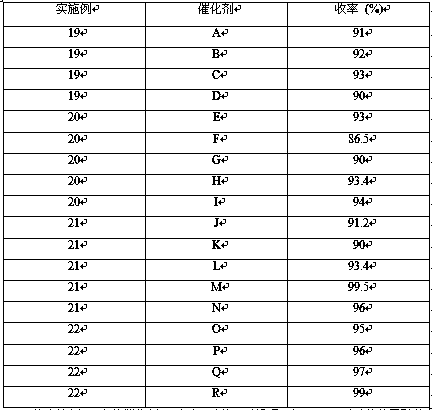
Method for preparing p-anisidine through catalytic hydrogenation by industrial-scale device
ActiveCN105294456AQuick responseHigh purityOrganic compound preparationAmino-hyroxy compound preparationP-nitroanisoleP-Anisidine
The invention relates a method for preparing p-anisidine through catalytic hydrogenation by an industrial-scale device. The method comprises the following steps: adding a raw material namely p-nitroanisole in an industrial-scale hydrogenation kettle in each production cycle in a continuous mode or an intermittent mode, and monitoring the concentrations of the raw material and impurities in the hydrogenation kettle in a reaction process in real time so as to obtain p-anisidine with the purity of 99.5% or above and yield of 100%. The purity of the p-anisidine product obtained through the method reaches 99.5% or above, so that p-anisidine can be directly used and sold; p-anisidine can further be subjected to rectification treatment to obtain a high-quality p-anisidine product with the purity of 99.9% or above which can be used in special fields.
Owner:NINGXIA ZHONGSHENG NEW TECH CO LTD
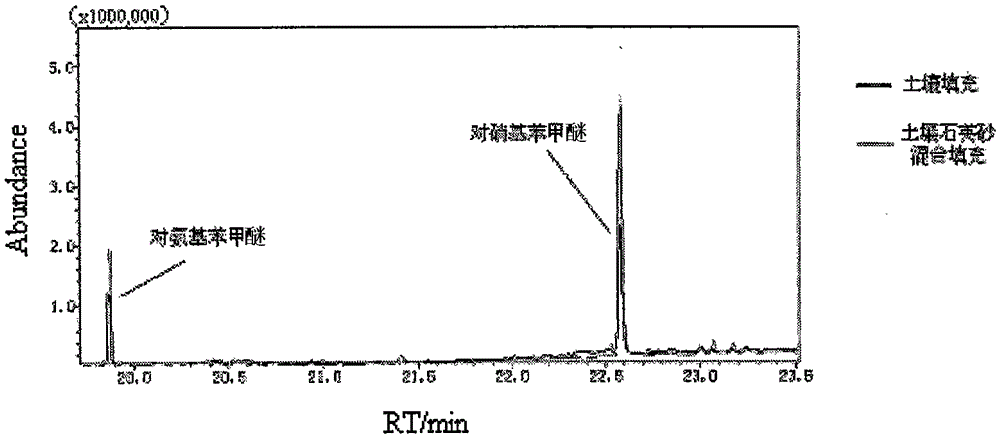
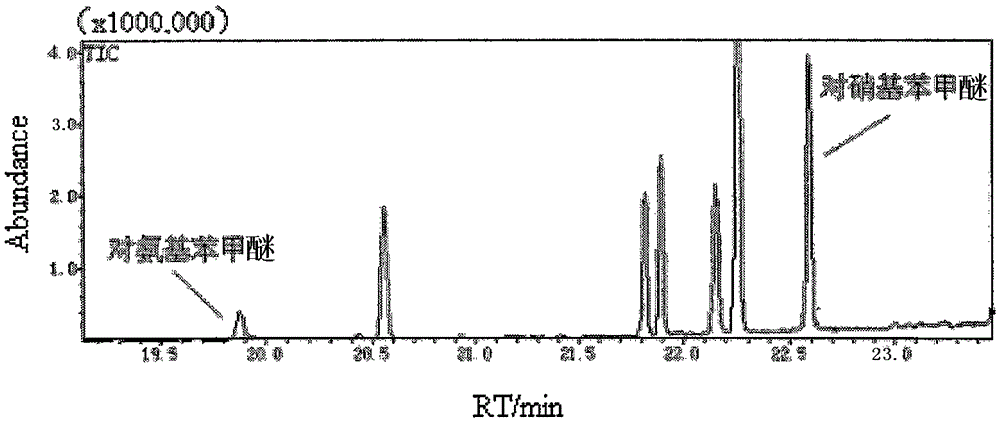
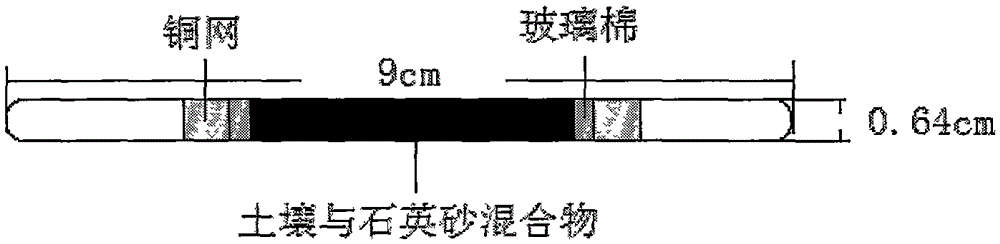
Method for detecting residual quantity of aminoanisole and paranitroanisole in soil
ActiveCN105467040AOvercome problems such as high temperature hardeningImprove detection efficiencyComponent separationP-nitroanisoleVapor phase chromatography
Owner:URUMCHI PONY TESTING TECH CO LTD
Synthesis method of 2-amino-4-acetamino anisole
ActiveCN107903182AHigh purityNo pollution in the processOrganic compound preparationCarboxylic acid amides preparationP-nitroanisoleHydrogen
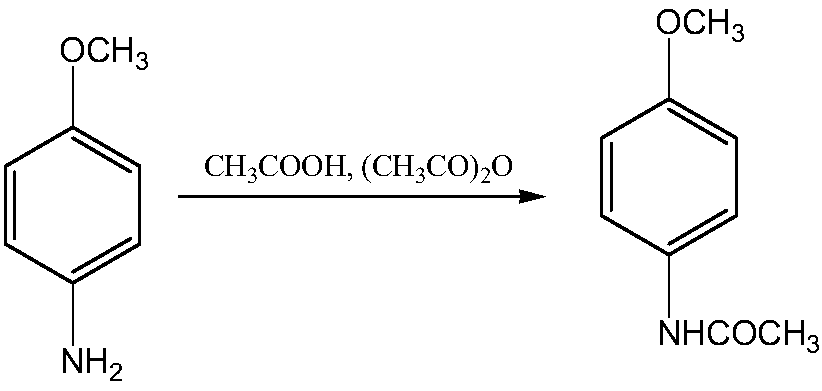
The invention discloses a synthesis method of 2-amino-4-acetamino anisole. The synthesis method comprises the following steps: (1) carrying out a primary catalytic hydrogenation reduction reaction onp-nitroanisole and hydrogen in the presence of a catalyst A to obtain p-methoxyaniline; (2) carrying out an acetylation reaction on the p-methoxyaniline to obtain p-acetanisidine; (3) carrying out a nitration reaction on the p-acetanisidine to obtain 2-nitro-4-acetamino anisole; and (4) carrying out a secondary catalytic hydrogenation reduction reaction on the 2-nitro-4-acetamino anisole and hydrogen in the presence of a catalyst B to obtain 2-amino-4-acetamino anisole. 2-amino-4-acetamino anisole is synthesized by using a method sequentially comprising acylation, nitration and reduction, so that the problems that raw materials are easily oxidized, the amount of side reactions is large and a product is difficult to separate and low in purity in a traditional directional acylation process are avoided; and the purity of the product synthesized by using the method disclosed by the invention can reach 99.5%-99.8%.
Owner:NINGXIA ZHONGSHENG NEW TECH CO LTD
P-nitroanisole synthesis method
PendingCN111646904AHigh yieldImprove product qualityOrganic chemistryOrganic compound preparationP-nitroanisoleNitrophenol
The invention relates to a synthetic method of p-nitroanisole, which can effectively reduce the generation of by-products, avoid the refining process of the by-product p-nitrophenol and greatly improve the yield of p-nitroanisole; besides, a small amount of p-nitrophenol can be further reused in the synthetic steps of the invention in the subsequent wastewater treatment process. In addition, the p-nitroanisole obtained by the method disclosed by the invention has higher product quality.
Owner:ZHEJIANG RUNTU INST
Aluminum electrolytic capacitor
InactiveCN100426434CImprove reliabilityLow risk of fireLiquid electrolytic capacitorsCapacitor electrolytes/absorbentsElectrolytic agentNitro compound
The present invention aims to provide a highly reliable aluminum electrolytic capacitor which has no flash points and shows little change or degradation in external appearance and properties. The electrolytic capacitor of the invention comprises a capacitor as an electrode using an aluminum foil, a driving electrolyte having a water content of 20-90wt%, a bottom barrel type house of the capacitor receiving the driving electrolyte, and a sealing material which seals an opening part of the house. The driving electrolyte contains one or more nitro compound of 0.01% by weight selected from p-nitrophenol, m-nitrophenol, o-nitrophenol, p-nitrobenzoic acid, m-nitrobenzoic acid, o-nitrobenzoic acid, p-nitroanisole, m-dinitroanisole, p-dinitroanisole. The freezing point of the electrolytic solution is under -10 DEG C, the chlorine content of the sealing material is less than 300ppm to the weight of the sealing material.
Owner:PANASONIC CORP
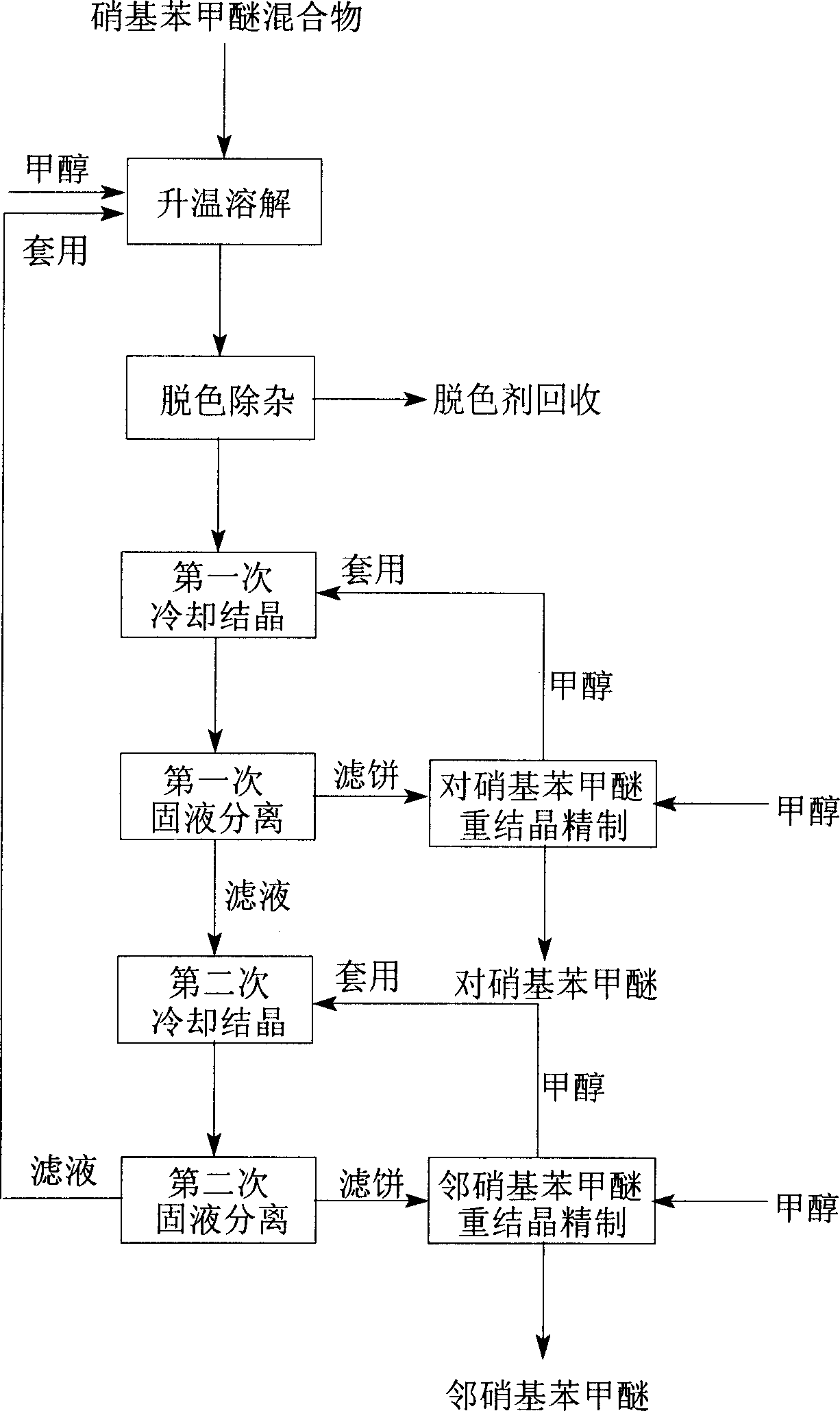
Crystallizing separating tech. of nitro methyl-phenoxide mixture
InactiveCN1861566AReduce energy consumptionAchieve separabilityOrganic chemistryOrganic compound preparationP-nitroanisoleO-Nitroanisole
A crystallizing process for separation of nitro phenylmether mixture includes such steps as thermal dissolving of said mixture in methanol, decoloring, removing impurities, filtering to obtain refined liquid, cooling step by step for crystallizing, filtering to obtain p-nitro phenylmether and o-nitro phenylmether, respectively recrystallizing in methol, separation and refining to obtain refined two products.
Owner:CHANGZHOU JIASEN CHEM +1
Method for preparing p-aminoanisole by catalytic hydrogenation
InactiveCN110407709AImprove performanceHigh strengthOrganic compound preparationHeterogenous catalyst chemical elementsP-nitroanisoleHydrogen atmosphere
The invention discloses a method for preparing p-aminoanisole by catalytic hydrogenation, and belongs to the technical field of catalytic hydrogenation. A p-nitroanisole solution is taken as a raw material, catalytic hydrogenation reaction is carried out in a trickle bed reactor by using a supported metallic nickel catalyst under a hydrogen atmosphere of 313-343 K and 1.0-2.0 MPa, on-line operation lasts for more than 4500 h, the conversion rate of the p-nitroanisole is greater than 99%, and the selectivity of the p-aminoanisole is greater than 99%.
Owner:NANJING UNIV
Method for preparing p-aminoanisole by catalytic hydrogenation of an industrial scale device
ActiveCN105294456BQuick responseHigh purityOrganic compound preparationAmino-hyroxy compound preparationP-nitroanisoleDistillation
The invention relates to a method for preparing p-aminoanisole by catalytic hydrogenation of an industrial-scale device, which is to add raw materials to p-aminoanisole continuously or intermittently during each production cycle in an industrial-scale hydrogenation kettle. Nitroanisole, and real-time monitoring of the concentration of raw materials and impurities in the hydrogenation tank during the reaction to obtain p-aminoanisole with a purity of more than 99.5% and a yield of 100%. The p-aminoanisole product obtained by the present invention has a purity of more than 99.5%, and can be used and sold directly; it can also be further rectified to obtain a p-aminoanisole product with a purity of more than 99.9%, which can be used in special fields.
Owner:NINGXIA ZHONGSHENG NEW TECH CO LTD
Preparation method of Iguratimod intermediate
PendingCN114539104AEasy to getPromote environmental protectionOrganic compound preparationSulfonic acid amide preparationP-nitroanisolePtru catalyst
The invention discloses a preparation method of an Iguratimod intermediate, which comprises the following steps: by taking p-nitroanisole as a raw material, carrying out substitution nucleophilic substitution (VNS) on p-nitroanisole and methoxyamine hydrochloride in the presence of a copper salt catalyst and an acid-binding agent to generate 5-methoxy-2-nitroaniline (compound II); the synthesis method comprises the following steps: carrying out a nucleophilic substitution reaction on 5-methoxy-2-nitroaniline (compound II) and methanesulfonyl chloride to generate a compound III, etherifying the compound III and phenol under the catalysis of a copper salt to generate N-(5-methoxy-2-phenoxy phenyl) methane sulfonamide (compound IV), and reagents used in the synthesis process are non-highly toxic products and are easy to obtain; no iron powder is used in the reaction process, so that iron mud which is harmful to the environment is not generated, and the environmental protection property is high; the reaction operation difficulty is small, the safety is high, and a foundation is laid for industrial preparation of Iguratimod drugs.
Owner:常州佳德医药科技有限公司
Preparation method of 4-(trifluoromethylthio)nitrobenzene
ActiveCN104610107AConducive to industrial mass productionFew reaction stepsSulfide preparationHydrogen fluorideP-nitroanisole
The invention provides a preparation method of 4-(trifluoromethylthio)nitrobenzene, which is implemented by reacting paranitroanisole with trifluoro-methanthiol at a temperature of 60-150 DEG C, so that 4-(trifluoromethylthio)nitrobenzene is obtained. According to the invention, products are prepared by adopting a new synthetic method, especially by taking new raw materials through a one-step process, the whole production process is normally operated, reaction conditions are mild, and the utilization of anhydrous hydrogen fluoride with large toxicity and corrosivity is effectively avoided, therefore, the method has a broad application prospect.
Owner:JIANGSU LINGYUN PHARMA
Preparation method of P-aminoanisole
InactiveCN108129336AReduce lossReduce pollutionOrganic compound preparationChemical recyclingP-nitroanisoleFiltration
The invention discloses a preparation method of P-aminoanisole. The p-aminoanisole is obtained through performing continuous catalytic hydrogenation on paranitroanisole and then sedimentation and membrane filtration. The preparation method disclosed by the invention can achieve the effects of high production efficiency, high product quality, low labor intensity, environmental friendliness, high catalyst utilization rate and beneficial industrialization.
Owner:YANTAI ANOKY FINE CHEM CO LTD +4
A kind of clean production method of p-aminoanisole
ActiveCN106187786BReduce generationAchieve zero emissionsOrganic compound preparationAmino-hyroxy compound preparationP-nitroanisoleAfter treatment
The invention discloses a clean production method of p-anisidine. The clean production method comprises the following steps of (1) enabling nitrochlorobenzene, sodium hydroxide and methanol to be subjected to an etherification reaction so as to obtain etherification reaction liquid; (2) directly filtering the etherification reaction liquid obtained in the step (1) to obtain a filtrate and filter cakes, washing the filter cakes with the methanol, drying the washed filter cakes to obtain industrial-grade sodium chloride, and distilling the filtrate so as to obtain p-nitroanisole concentration liquid; and (3) under the condition that a hydrogenation catalyst exists, enabling the p-nitroanisole concentration liquid obtained in the step (2) to be subjected to a hydrogenation reduction reaction in the hydrogen atmosphere, and after the reaction is completed, performing after-treatment so as to obtain the p-anisidine. According to the clean production method, after the etherification reaction is finished, direction filtration is performed so that the sodium chloride is removed, then concentration treatment is performed on the etherification reaction liquid, the hydrogenation reaction is directly performed, the operation is simple and convenient, and the yield and the purity of the obtained product are high.
Owner:ZHEJIANG RUNTU INST
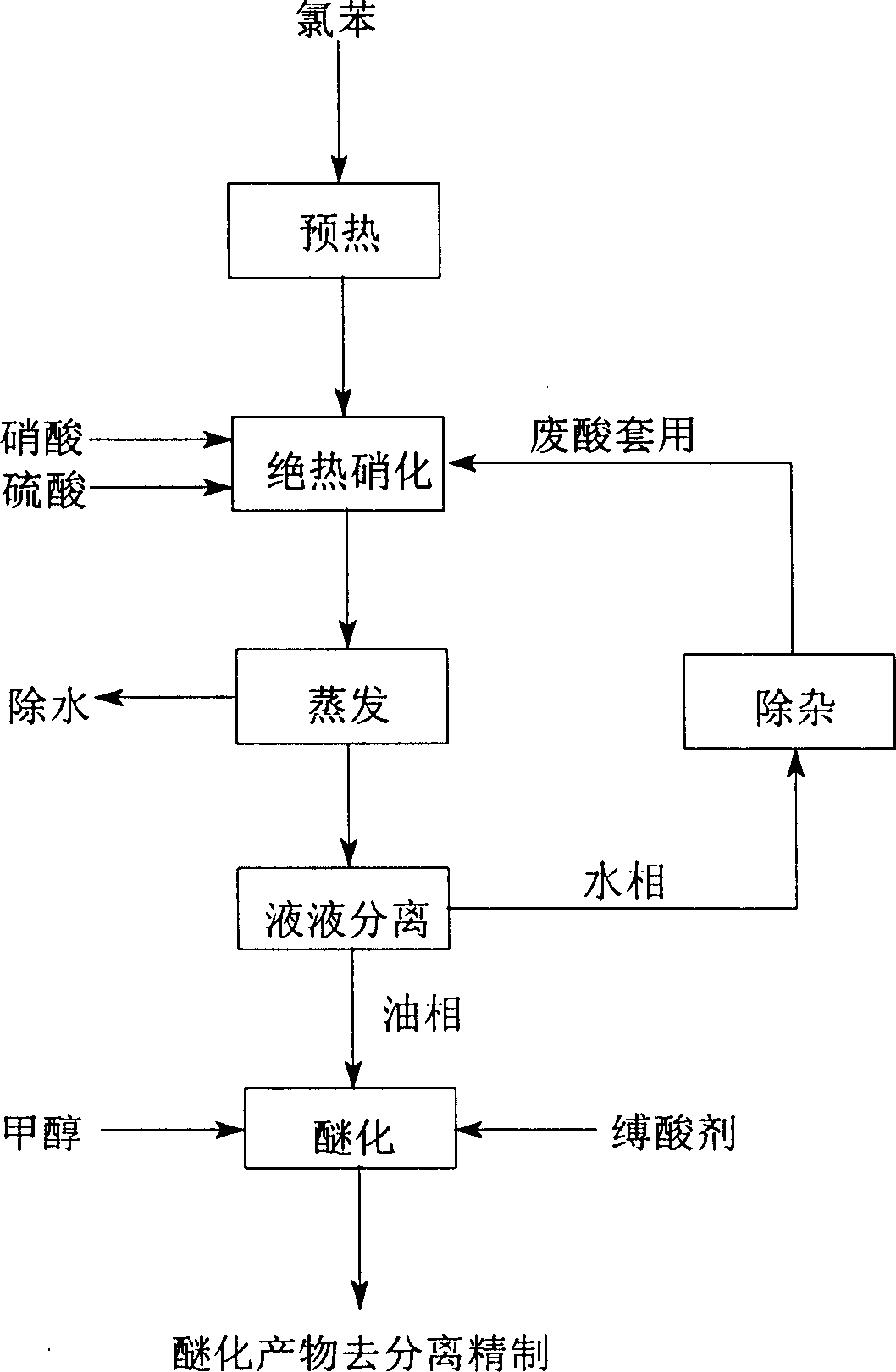
Tech. of producing ortho nitro methyl-phenoxide para nitro methyl-phenoxide and meta nitro chlorobenzene from chlorobenzene
InactiveCN100368377CReduce manufacturing costImprove production stabilityNitro compound preparationChlorobenzeneP-nitroanisole
A process for preparing o-nitro phenylmether, p-nitro phenylmether and meta-nitro chlorobenzene from chlorobenzene includes such steps as nitrifying reaction to obtain the nitro chlorobenzene mixture, etherifying reaction in etherifying agent, separating, and refining to obtain said products.
Owner:CHANGZHOU JIASEN CHEM +1
Preparation method of N-methyl-4-methoxyaniline
ActiveCN109503398AImprove performanceReduce the difficulty of separationOrganic compound preparationAmino-hyroxy compound preparationP-nitroanisoleHydrogen atmosphere
The invention provides a preparation method of N-methyl-4-methoxyaniline. The method comprises the following steps: mixing p-nitroanisole, paraformaldehyde and a catalyst, and carrying out a reductionreaction in a hydrogen atmosphere to obtain the N-methyl- 4-methoxyaniline. The preparation method provided by the invention is few in steps, simple to operate, mild in conditions, stable in catalystperformance and low in cost. No solvent is required, and the separation difficulty of a product is reduced. According to the description of embodiments, the product yield of the preparation method is86% or more than 86 %.
Owner:江苏智远科创科技有限公司 +2
Preparation method of p-anisidine
PendingCN113024388AEffective hydrogen absorptionConvenient dosageOrganic compound preparationChemical recyclingChemical synthesisP-nitroanisole
The invention provides a preparation method of p-anisidine, and belongs to the technical field of chemical synthesis. The method comprises the following steps: firstly, adding a predetermined amount of methanol and a catalyst into a hydrogenation kettle, replacing, removing oxygen and activating the catalyst, and dropwise adding a raw material p-nitroanisole into the hydrogenation kettle at a predetermined hydrogen pressure and a predetermined reaction temperature to carry out a hydrogenation reaction. According to the method, in the process of reducing the use amount of methanol and dropwise adding p-nitroanisole for hydrogenation reaction, the concentration of p-nitroanisole in a hydrogenation kettle is always kept at a lower level, the solvent and the catalyst are sufficient, p-aminoanisole which is continuously generated is utilized to play a role of the solvent, the substrate reaction is more thorough, the reaction time is greatly shortened, and the generation amount of byproducts is reduced. Experiments show that the single-kettle hydrogenation reaction time is shortened by 30%-47%, the single-kettle productivity can be improved by 33%-97%, meanwhile, the single-kettle reaction time is shortened, the daily productivity can be improved by 77%-125%, and the purity of p-anisidine in the product reaches 99.3%-99.7%.
Owner:宁夏华御化工有限公司
A kind of method that p-nitroanisole catalytic hydrogenation prepares p-aminoanisole
ActiveCN108047064BHigh activityHigh yieldMolecular sieve catalystsOrganic compound preparationPtru catalystP-nitroanisole
The invention discloses a method for preparing p-anisidine through catalytic hydrogenation of p-nitroanisole. According to the method, p-nitroanisole is used as a reaction raw material and subjected to a reaction in an enclosed reactor with a mechanical stirring function under the conditions of usage of a composite catalyst, no usage of a solvent, a reaction temperature of 80 to 150 DEG C and hydrogen pressure of 0.3 to 1 MPa so as to obtain p-anisidine, wherein the composite catalyst is composed of active components metal M and a carrier; the active components metal M are composed of two or more selected from a group consisting of Cu, Ni, Fe, Zn, Co, Cr and Mo, and the content of the active components metal M accounts for 0.3 to 25 wt% of the mass of the composite catalyst; and the carrier is prepared by compounding a carbon-containing organic matter and an inorganic oxide, a mass ratio of the carbon-containing organic matter to the inorganic oxide is 1: 50 to 1: 10, and the inorganicoxide is any one selected from a group consisting of SiO2, Al2O3, MgO, TiO2, CeO2 and a 4A molecular sieve. The composite catalyst used in the invention has high catalytic activity, stable performance and high selectivity and conversion rate.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of synthetic method of 2-amino-4-acetamidoanisole
ActiveCN107903182BHigh purityNo pollution in the processOrganic compound preparationCarboxylic acid amides preparationPtru catalystP-nitroanisole
The invention discloses a synthesis method of 2-amino-4-acetamino anisole. The synthesis method comprises the following steps: (1) carrying out a primary catalytic hydrogenation reduction reaction onp-nitroanisole and hydrogen in the presence of a catalyst A to obtain p-methoxyaniline; (2) carrying out an acetylation reaction on the p-methoxyaniline to obtain p-acetanisidine; (3) carrying out a nitration reaction on the p-acetanisidine to obtain 2-nitro-4-acetamino anisole; and (4) carrying out a secondary catalytic hydrogenation reduction reaction on the 2-nitro-4-acetamino anisole and hydrogen in the presence of a catalyst B to obtain 2-amino-4-acetamino anisole. 2-amino-4-acetamino anisole is synthesized by using a method sequentially comprising acylation, nitration and reduction, so that the problems that raw materials are easily oxidized, the amount of side reactions is large and a product is difficult to separate and low in purity in a traditional directional acylation process are avoided; and the purity of the product synthesized by using the method disclosed by the invention can reach 99.5%-99.8%.
Owner:NINGXIA ZHONGSHENG NEW TECH CO LTD
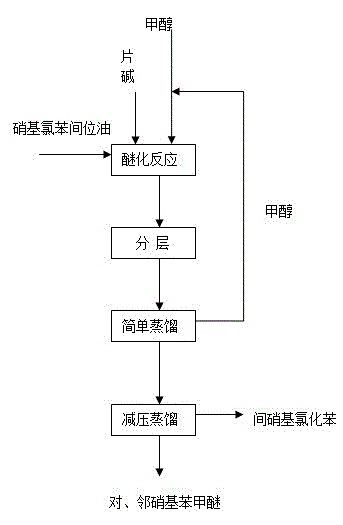
A kind of method preparing nitroanisole with nitrochlorobenzene meta-position oil
ActiveCN104557557BShort reaction timeShort reaction cycleOrganic chemistryOrganic compound preparationP-nitroanisoleO-Nitroanisole
The purpose of the present invention is to provide a method for producing nitroanisole with low cost, low energy consumption, short production cycle, and less waste, that is, using nitrochlorobenzene meta-oil under anhydrous system to prepare meta Methods for nitrochlorobenzene, p-nitroanisole, and o-nitroanisole. In order to achieve the above object, the technical scheme adopted in the present invention is as follows: nitrochlorobenzene meta-oil is added in the autoclave, and methanol and sodium hydroxide are added respectively, wherein the molar ratio of sodium hydroxide and meta-oil is 0.01 ~ 2.00 : 1, the molar ratio of methanol to meta-oil is 1~20:1, the reaction temperature is 10~200°C, the reaction time is 1~20 hours, the pressure is 0.1~4.0MPa, and the reaction product is analyzed by gas chromatography. Its content varies with the composition of the reaction raw material meta-oil. Compared with the traditional technology, the invention has the advantages of low production cost, short process, low energy consumption and little pollution.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com