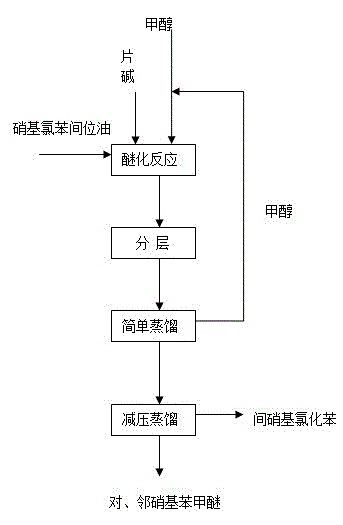
Method for preparing nitroanisole from m-nitrochlorobenzene oil
A technology for nitrochlorobenzene meta oil and nitroanisole, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc. Distillation energy consumption and other problems, to achieve the effect of short reaction time, reduce production costs, reduce process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 157.5 g (1 mol) of nitrochlorobenzene meta-oil, 240 ml (6 mol) of methanol, and 20 g (0.5 mol) of sodium hydroxide into the reaction kettle. Heat to 120°C, control the reaction pressure to 0.3MPa, and keep the reaction time for 3 hours. Sampling analysis showed that the total etherification conversion rate of o- and p-nitrochlorobenzene was 99.5%.
Embodiment 2
[0036] Embodiment 2 (comparative example)
[0037] Add 157.5 g (1 mol) of nitrochlorobenzene meta-oil, 240 ml (6 mol) of methanol, and 20 g (0.5 mol) of sodium hydroxide into the reaction kettle. Heating to methanol reflux temperature of 70°C, reflux reaction, keeping the reaction time for 10 hours. Sampling analysis showed that the total etherification conversion rate of o- and p-nitrochlorobenzene was 85.0%.
Embodiment 3
[0039] Add 157.5 g (1 mol) of meta-nitrochlorobenzene oil, 240 ml (6 mol) of methanol (recovered methanol) and 20 g (0.5 mol) of sodium hydroxide in the reaction kettle. Heat to 120°C, control the reaction pressure to 0.3MPa, and keep the reaction time for 3 hours. Sampling analysis showed that the total etherification conversion rate of o- and p-nitrochlorobenzene was 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com