Method for synthesizing para aminophenylmethylether by catalytic hydrogenation of paranitroanisole
A technology for p-nitroanisole and p-aminoanisole is applied in the field of catalyzing p-nitroanisole hydrogenation to synthesize p-aminoanisole with a supported palladium catalyst, and can solve the problem of waste disposal difficulties and high reaction temperature , short process flow and other problems, to achieve the effect of being conducive to environmental protection, mild reaction conditions, and clean reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
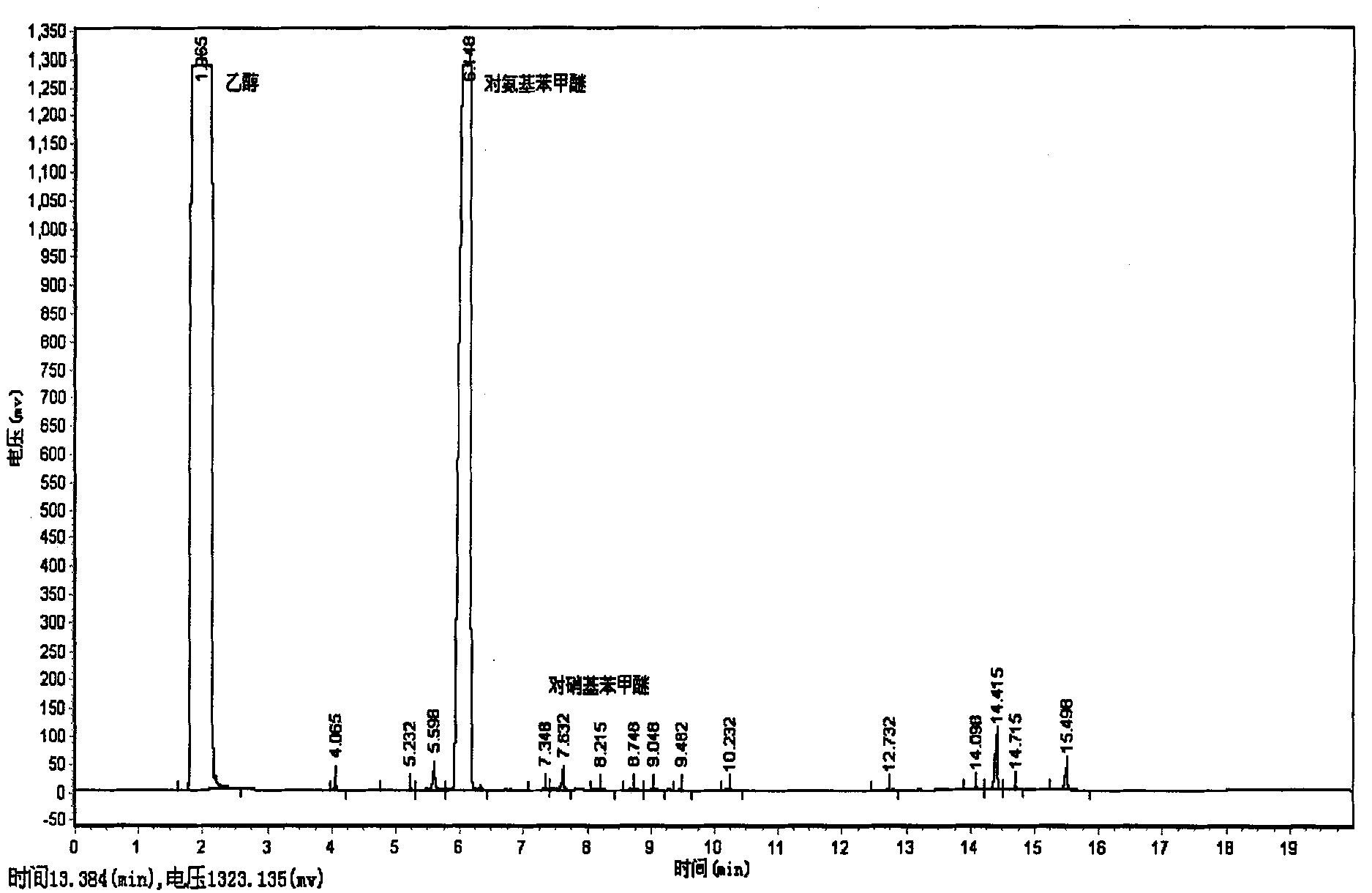
[0014] (1), add 0.306 gram of p-nitroanisole (analytical pure) and 0.0015 gram of Pd / C catalyst (the mass fraction of Pd is 5%) in 50 milliliters of autoclaves, at room temperature use carbon dioxide to sweep, discharge Air in the empty reaction system;
[0015] (2), the reactor is heated to 40 DEG C; Charge hydrogen into the reactor, and the pressure in the reactor is 2 MPa; Then charge into carbon dioxide, the pressure in the reactor is 14 MPa; Stir, and the reaction time is 10 MPa Minutes; After the reaction, naturally cool to room temperature, filter, and separate the product to obtain p-aminoanisole. The yield thereof was 25.51%.
Embodiment 2
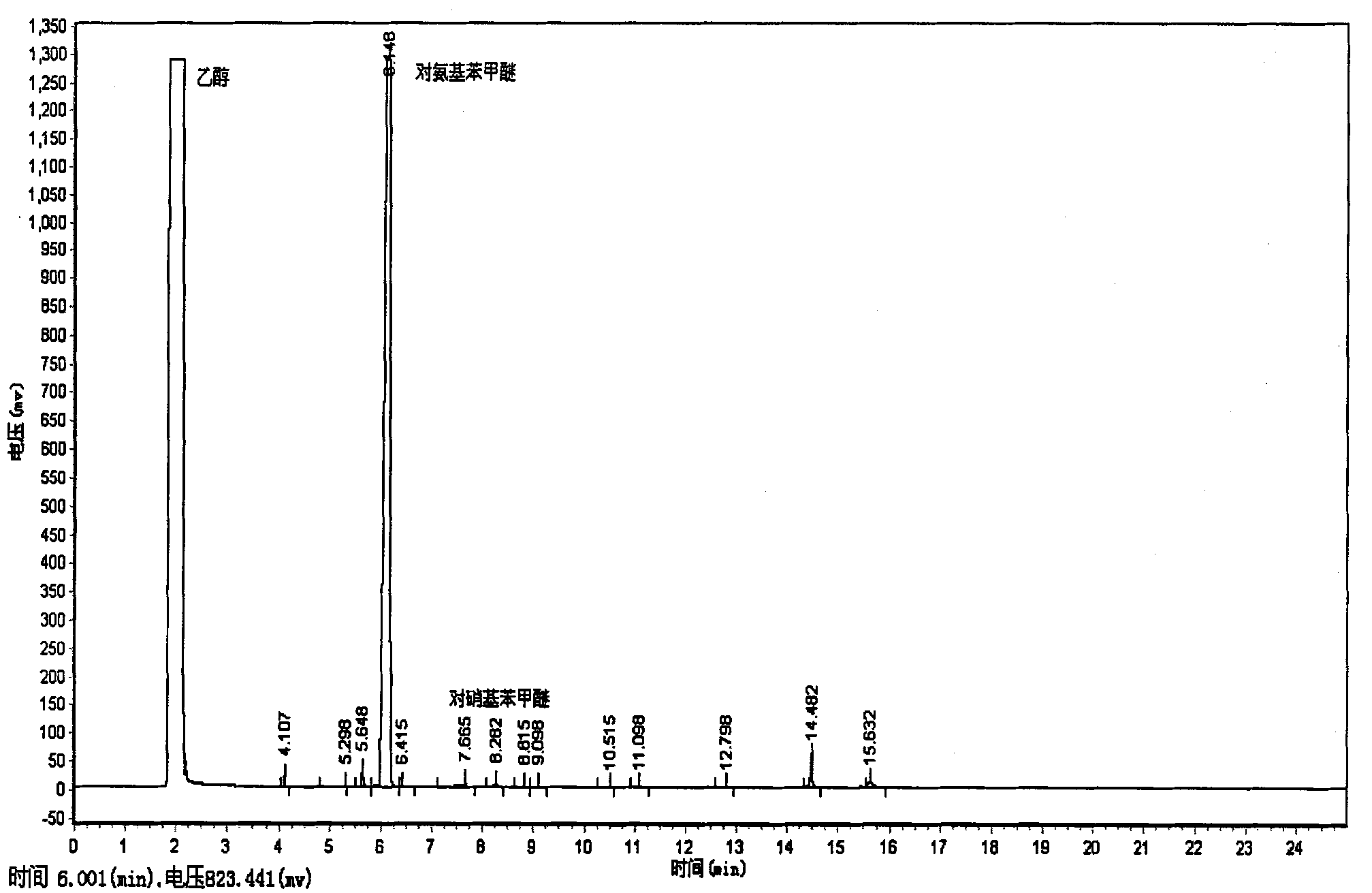
[0017] (1) with embodiment 1;
[0018] (2), the reactor is heated to 60 DEG C; Charge hydrogen into the reactor, and the pressure in the reactor is 4 MPa; Then charge into carbon dioxide, the pressure in the reactor is 16 MPa; Stir, and the reaction time is 10 MPa Minutes; After the reaction, naturally cool to room temperature, filter, and separate the product to obtain p-aminoanisole. The yield is 98.87%.
Embodiment 3
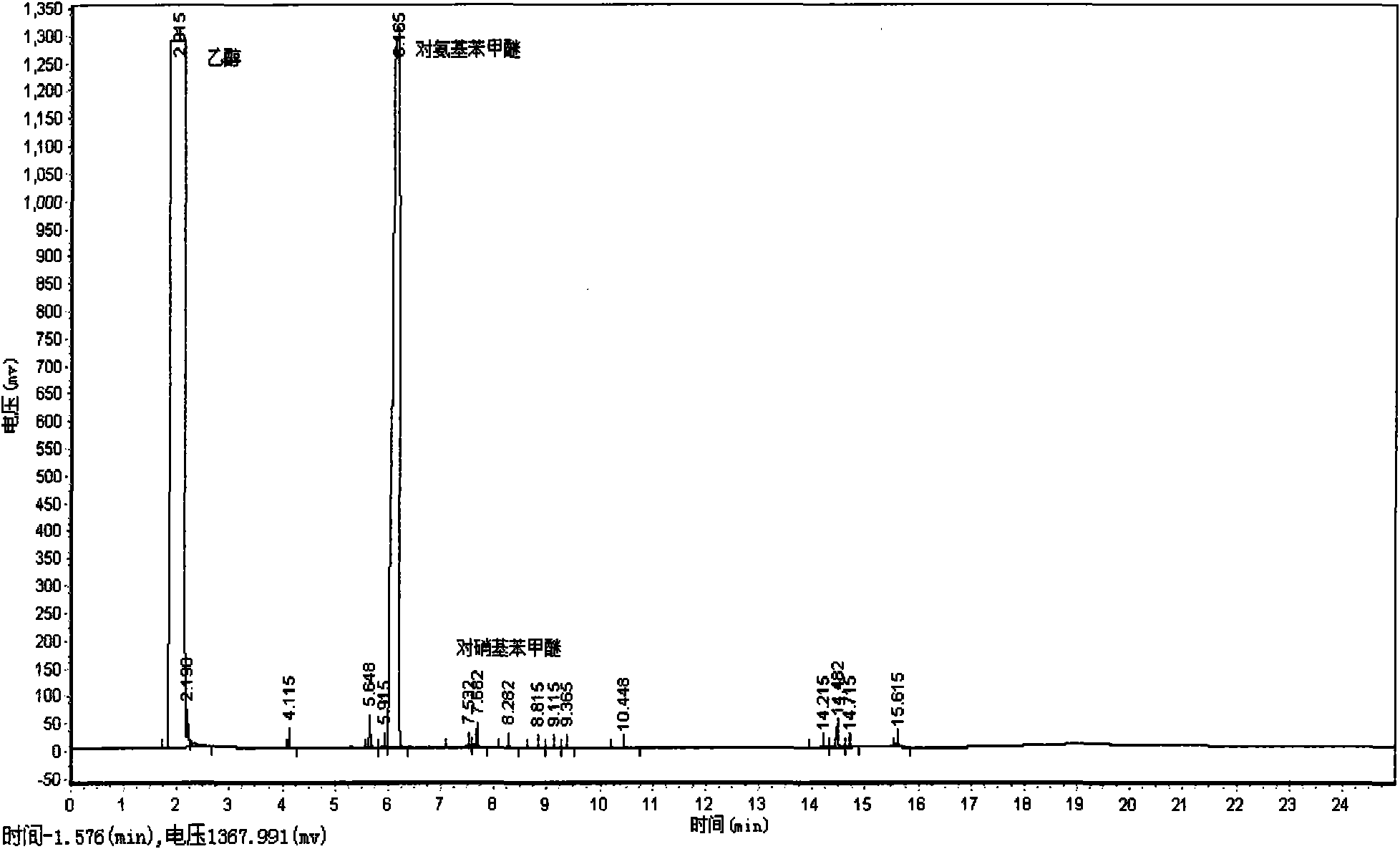
[0020] (1) with embodiment 1;
[0021] (2), the reaction kettle is heated to 60 ℃; In the reaction kettle, charge hydrogen, the pressure in the reaction kettle is 1 MPa; Then charge into carbon dioxide, the pressure in the reaction kettle is 13 MPa; Stir, the reaction time is 20 Minutes; after the reaction, naturally cool to room temperature, filter, isolate the product, and obtain p-aminoanisole yield of 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com