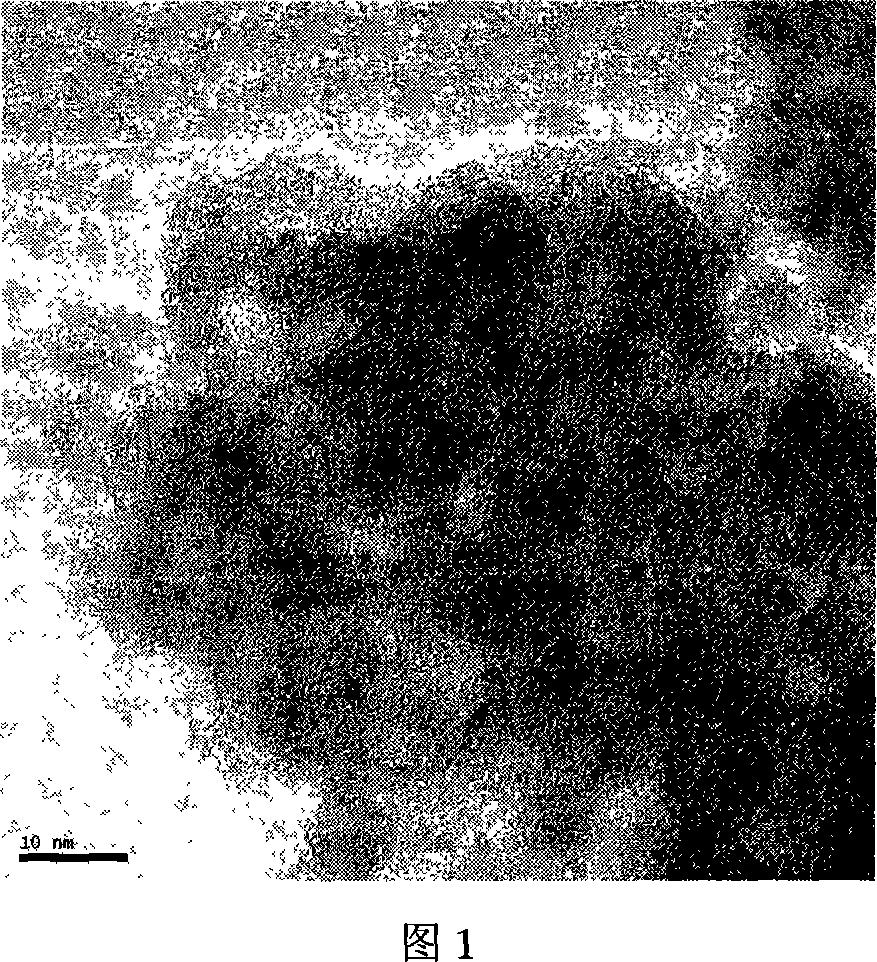
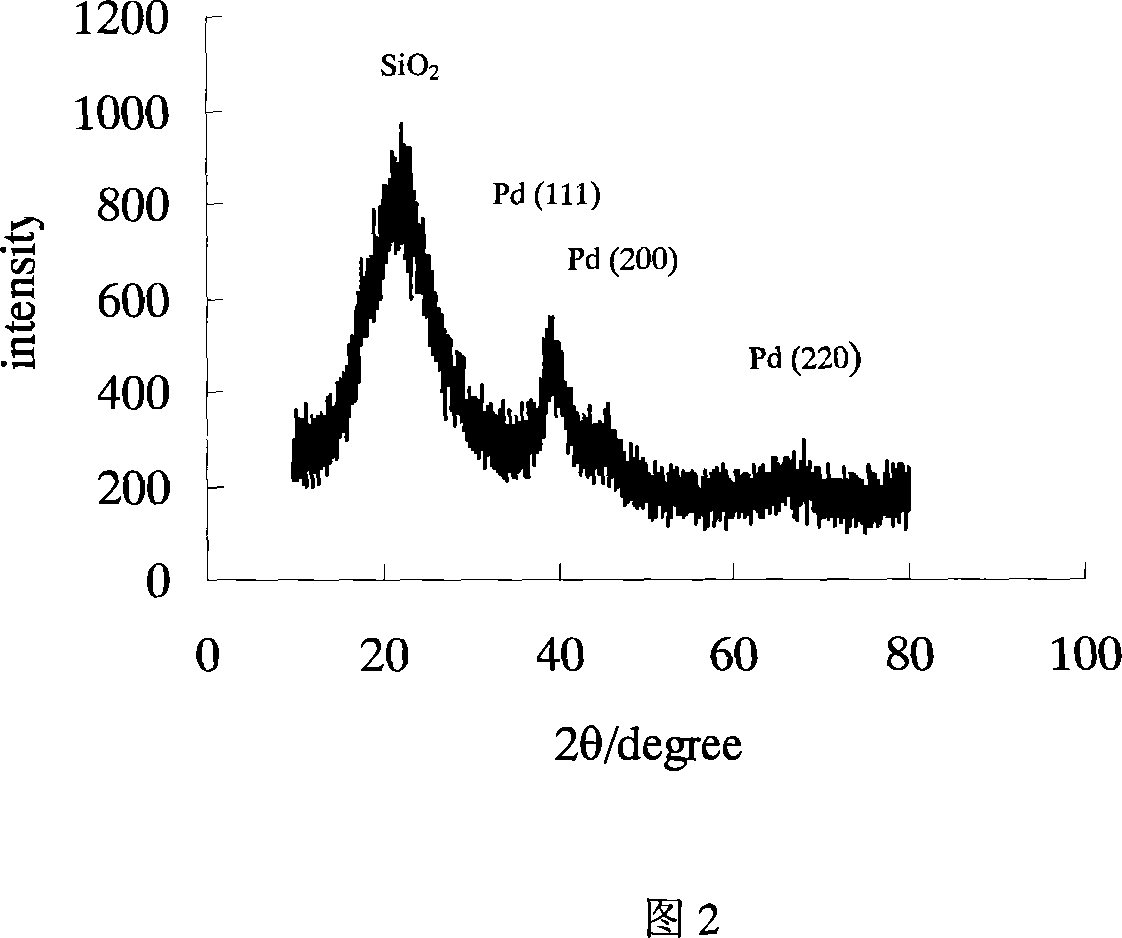
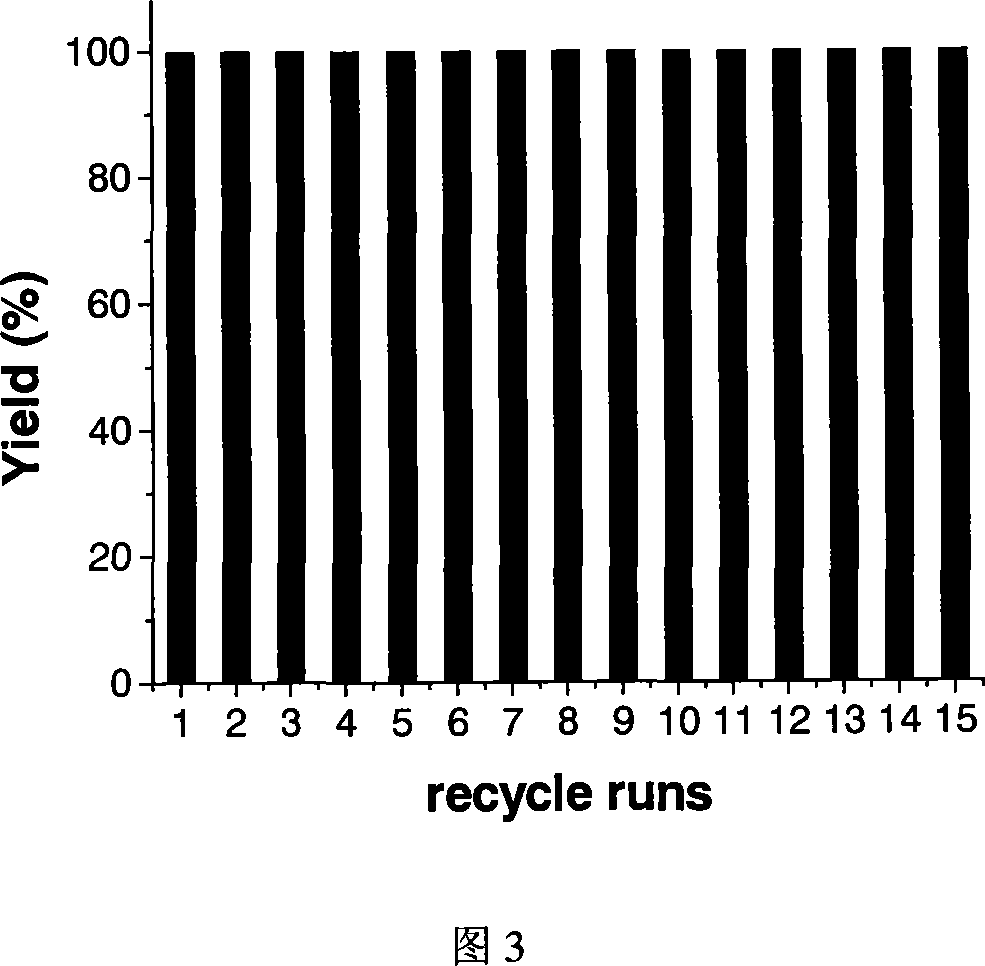
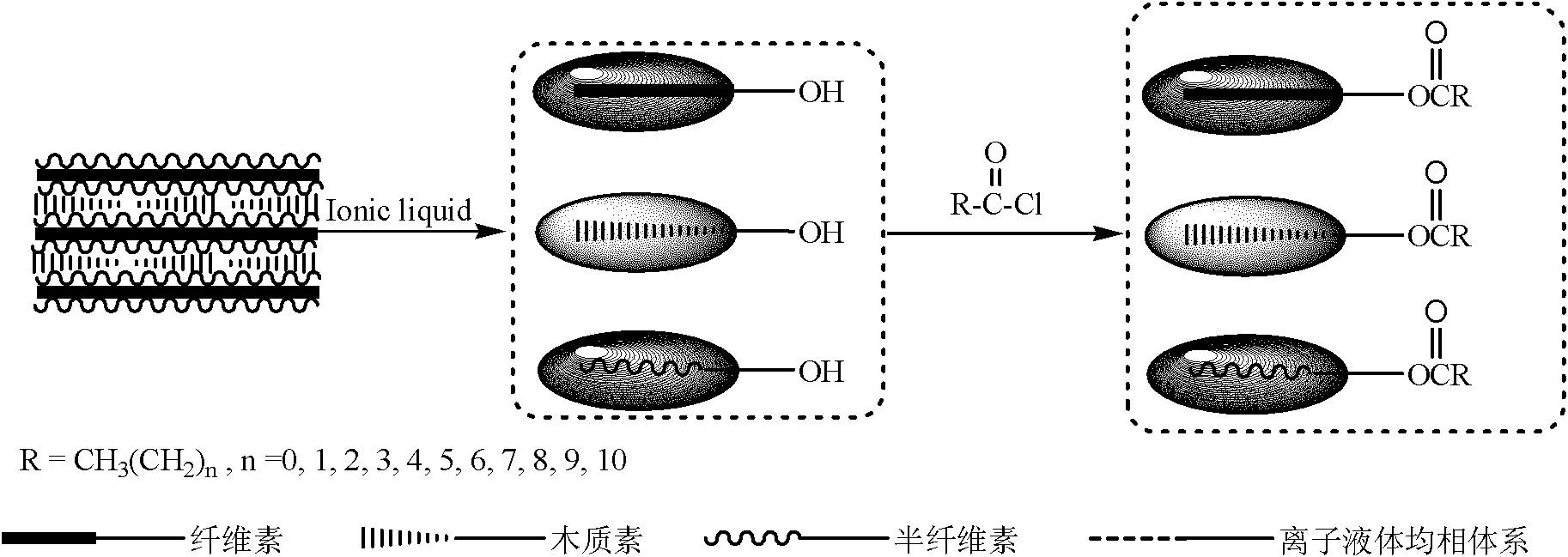
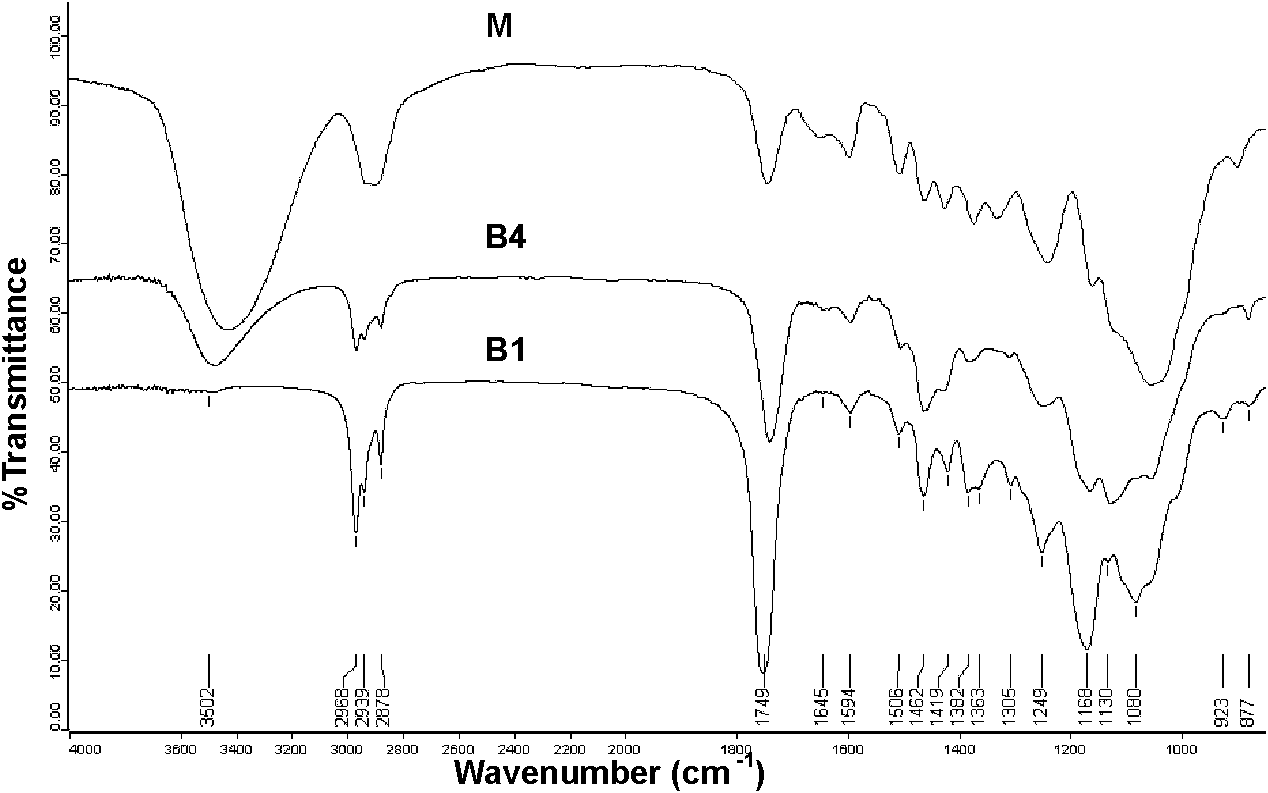
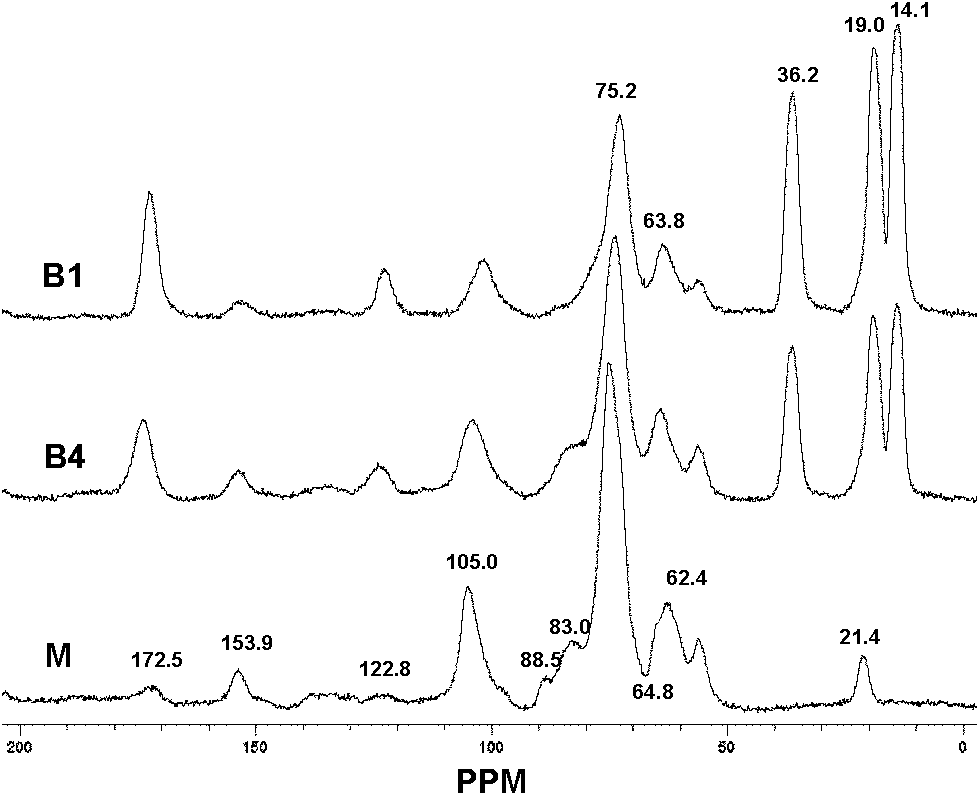
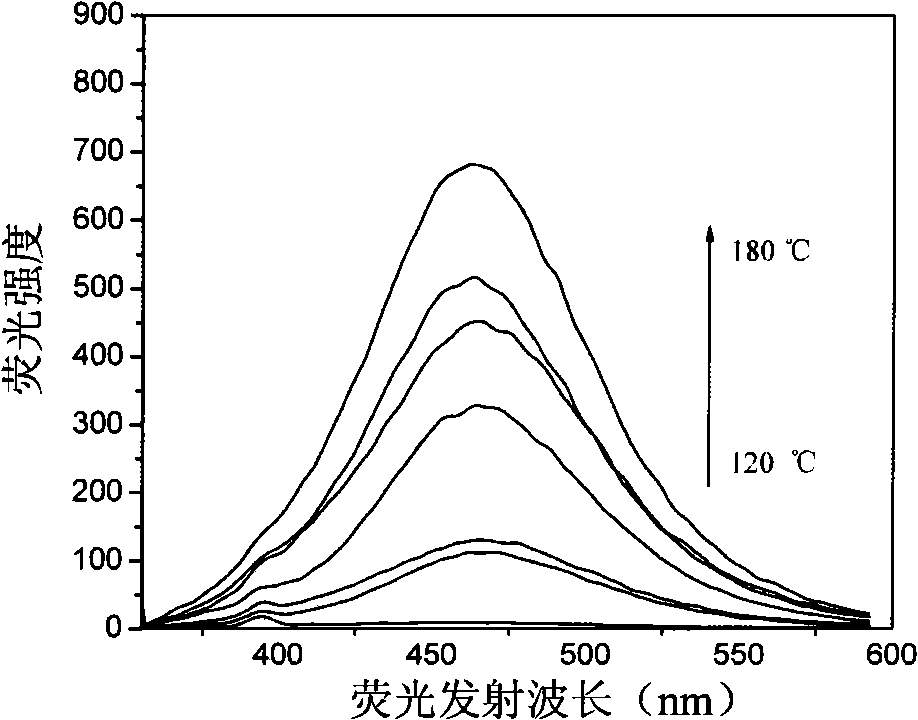
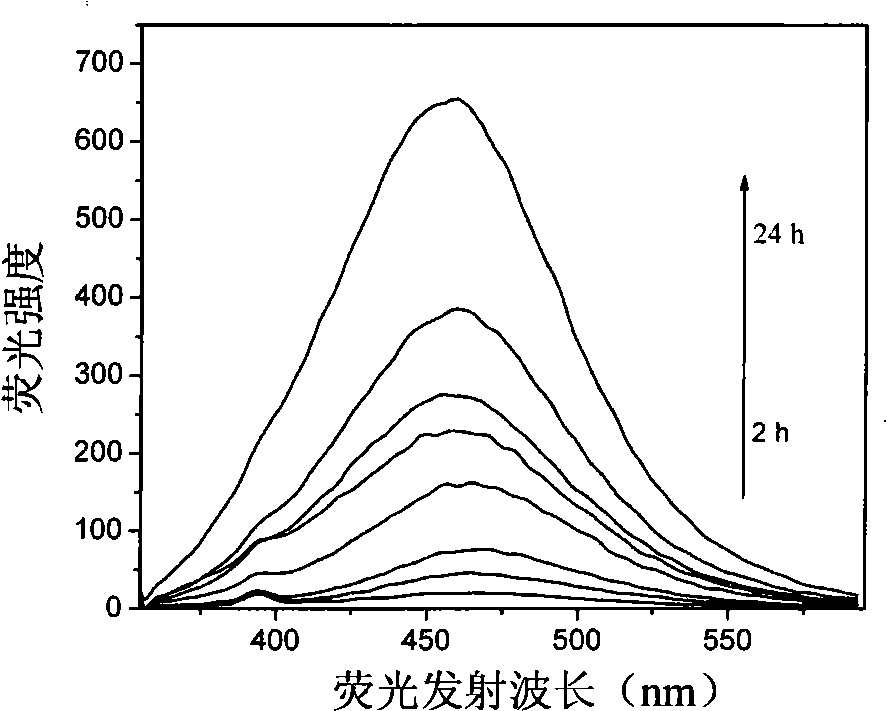
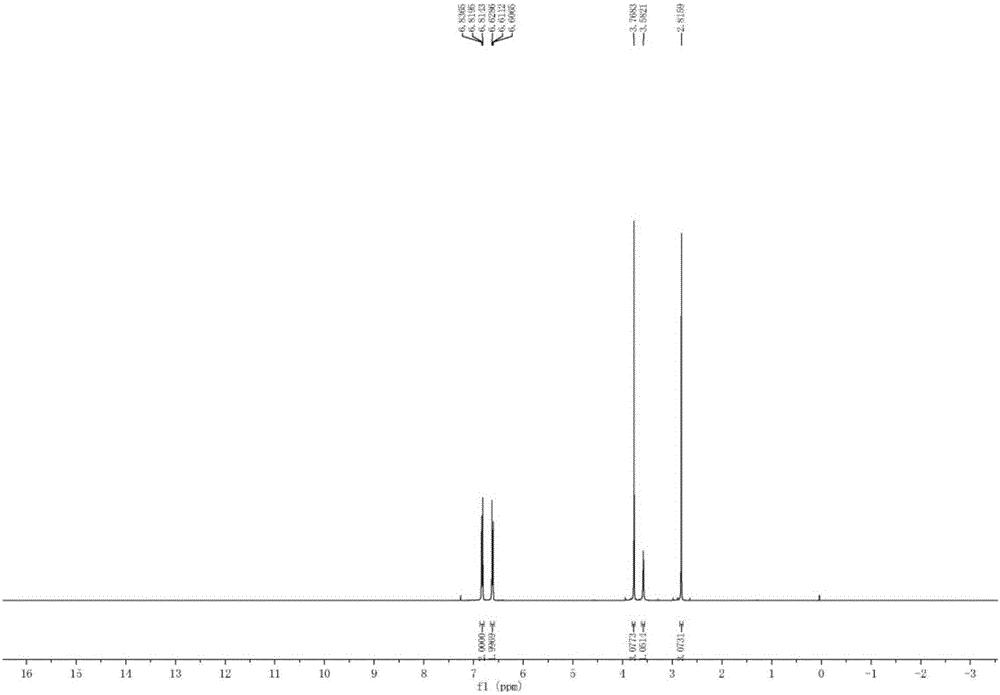
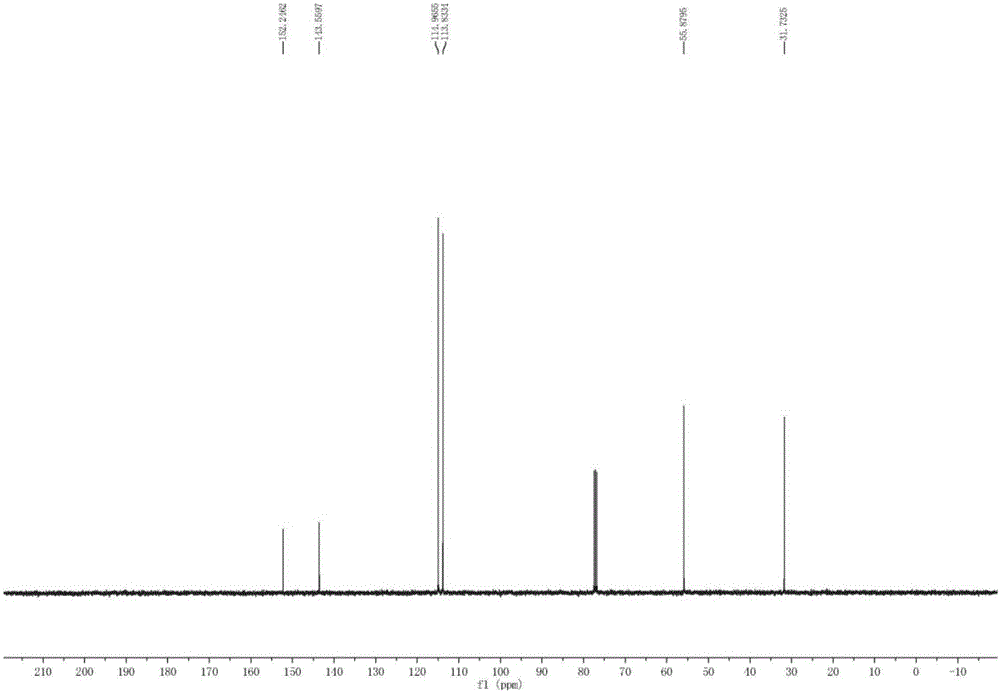
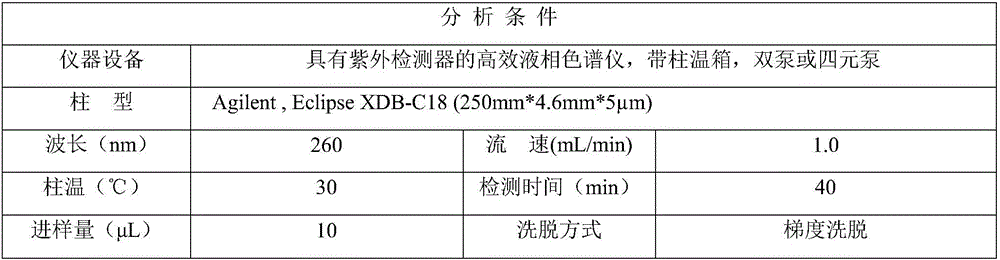
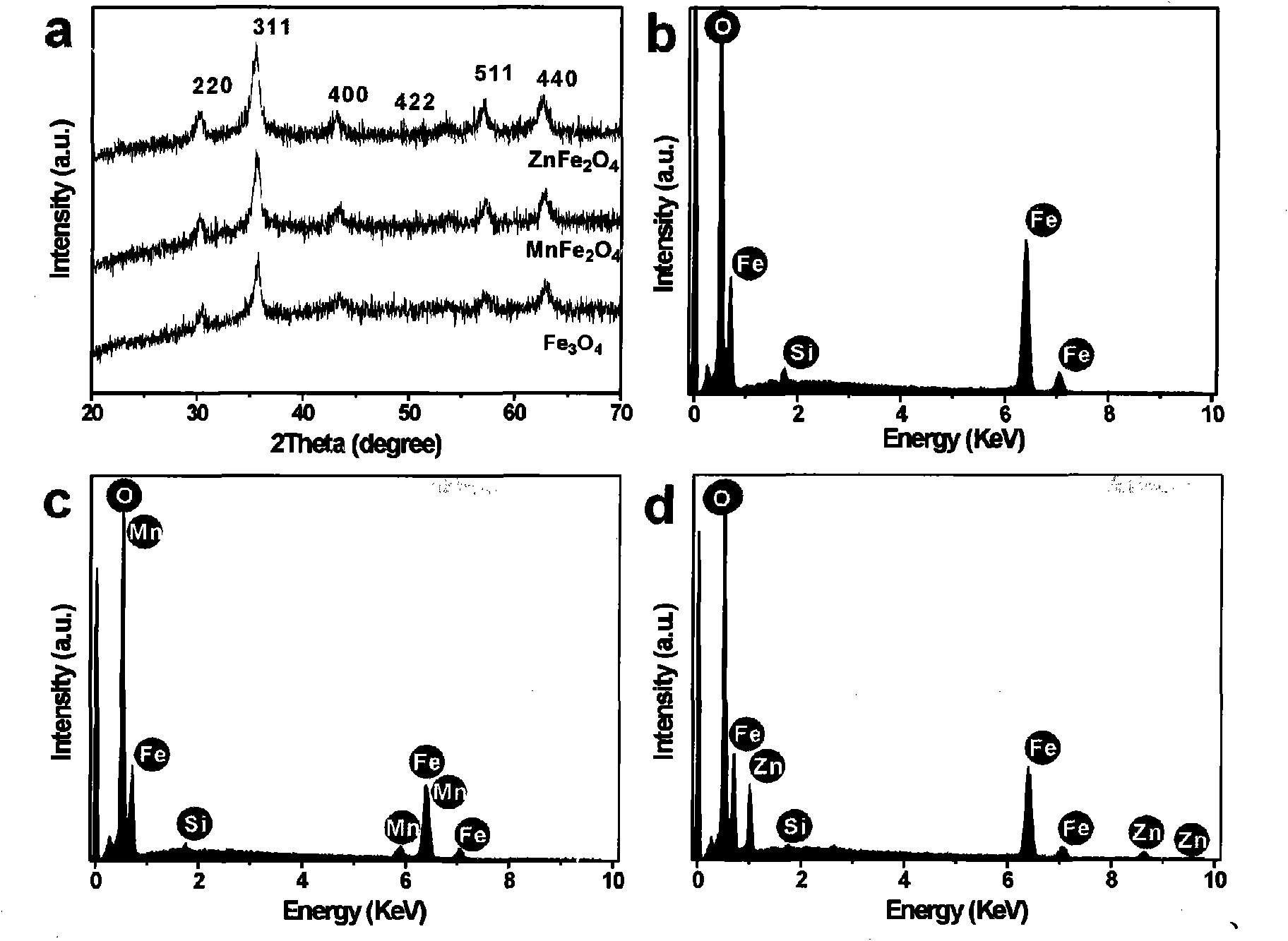
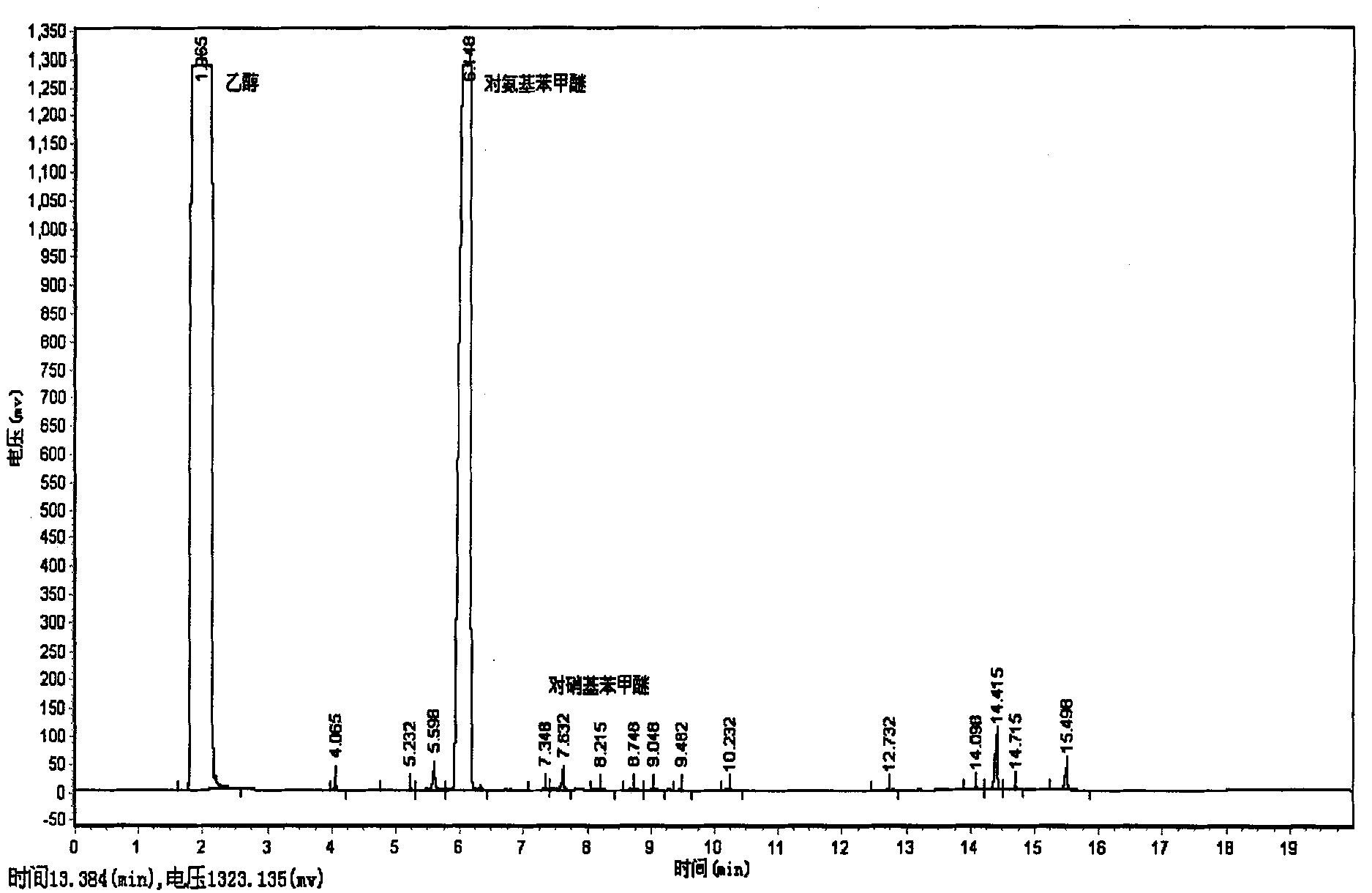
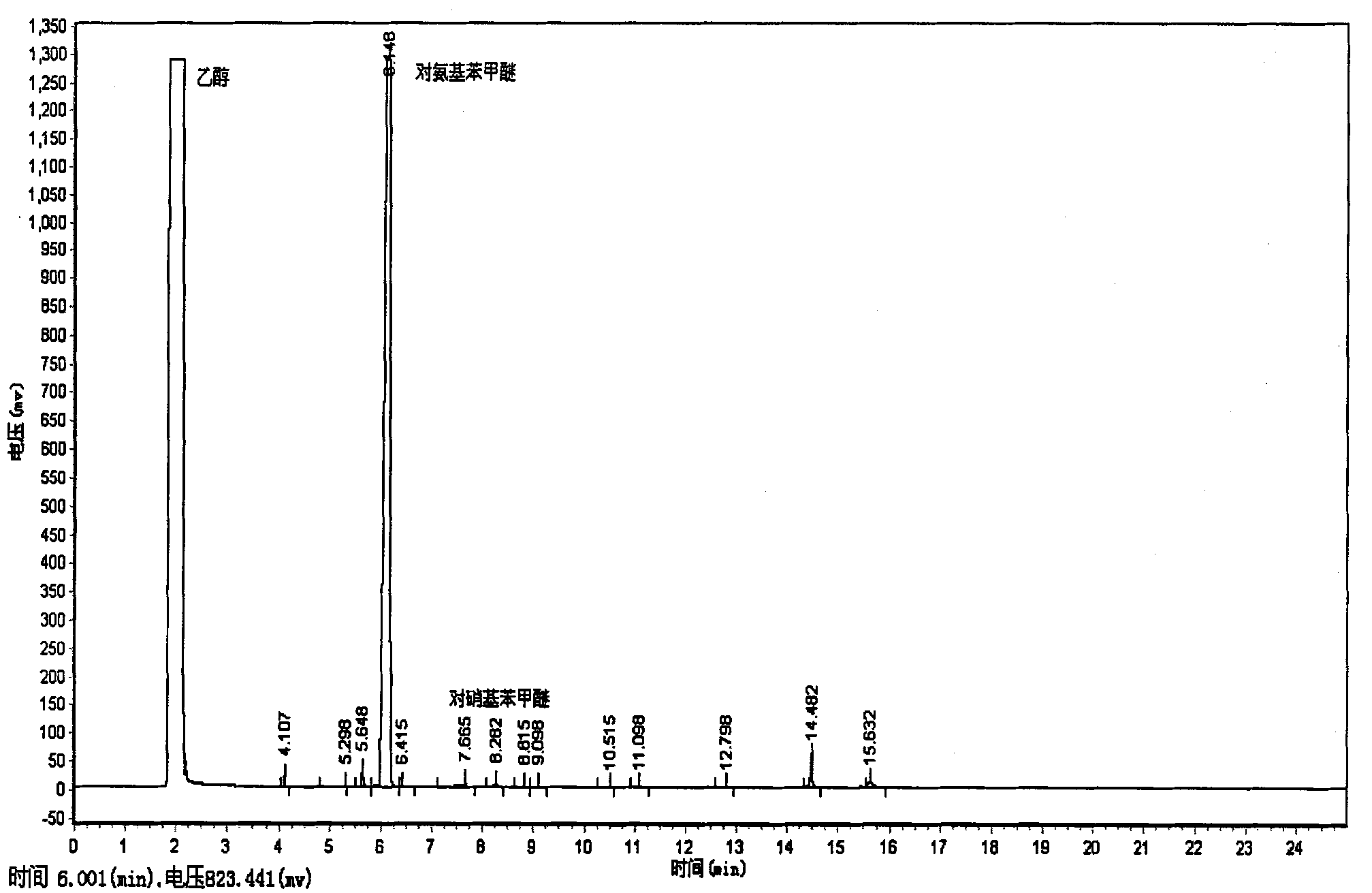
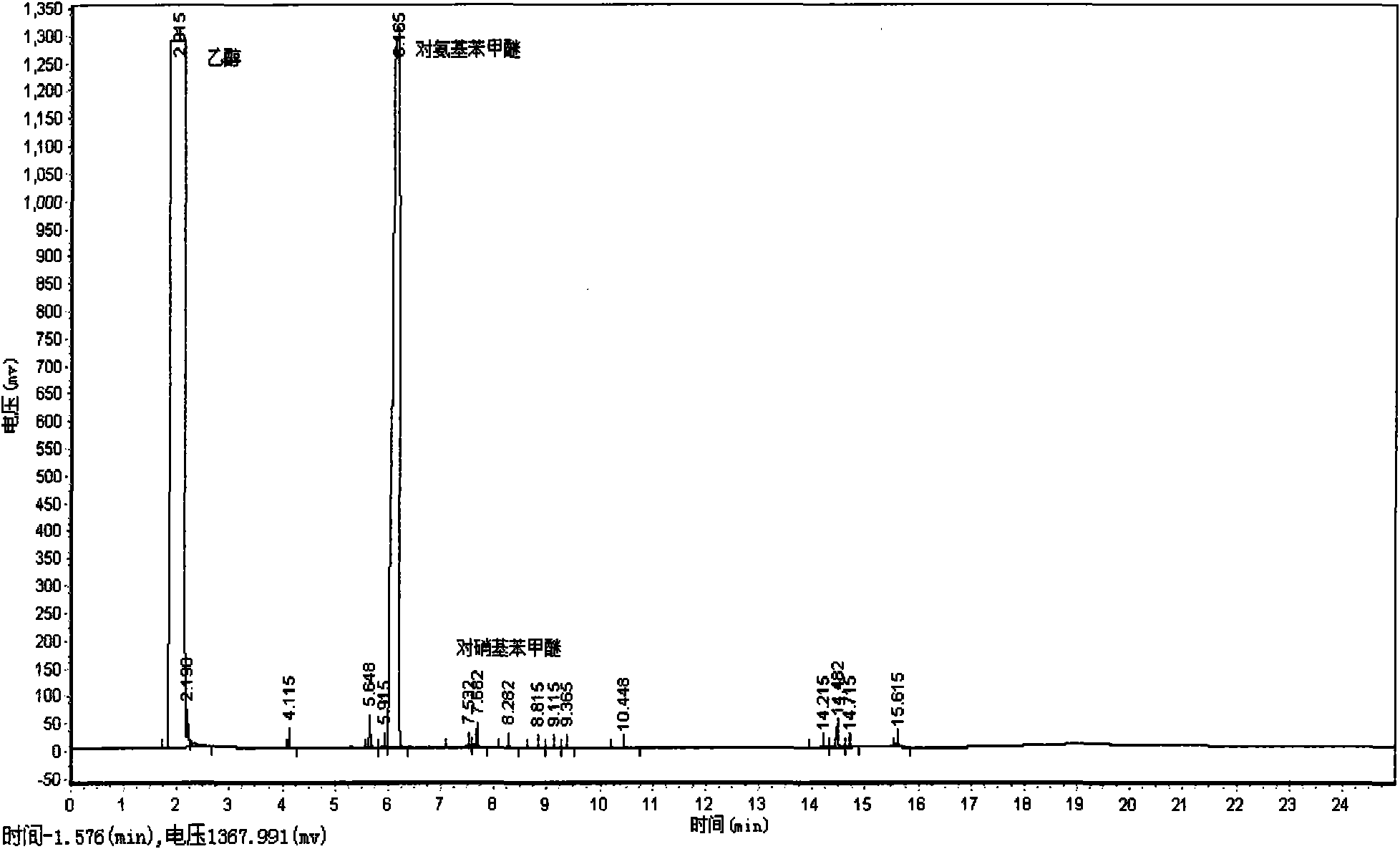
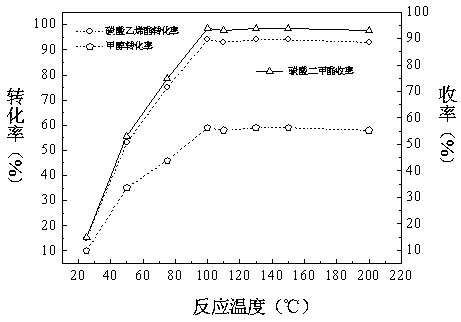
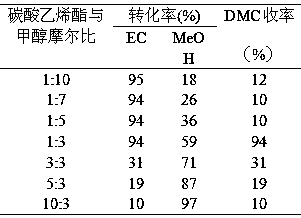
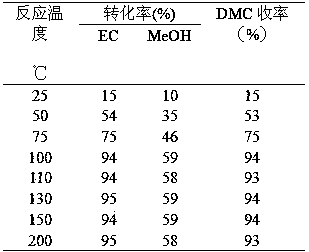
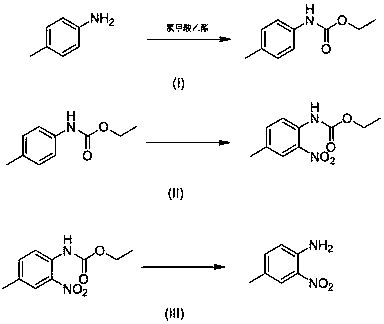
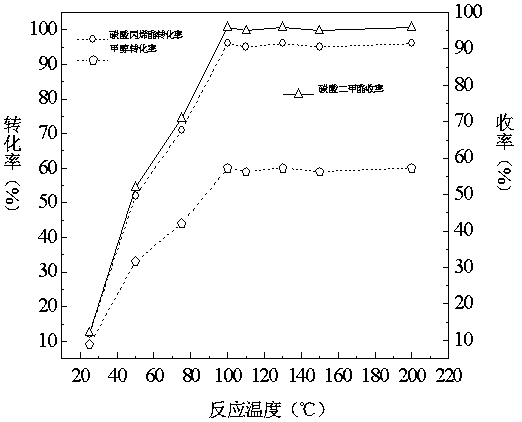
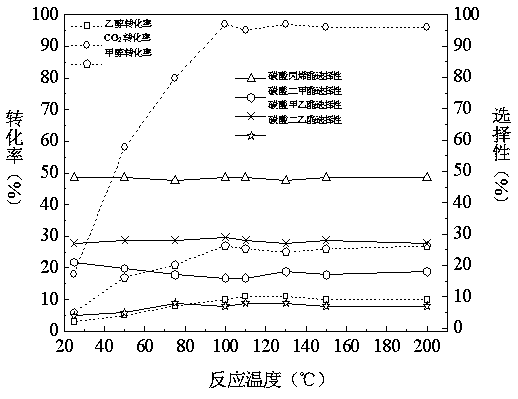
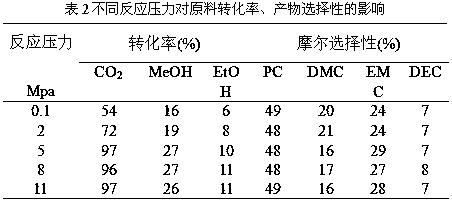
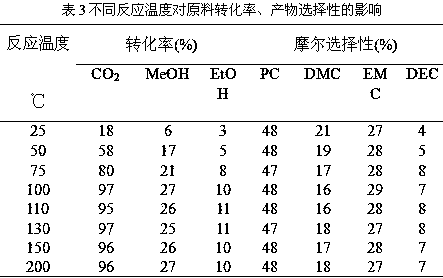
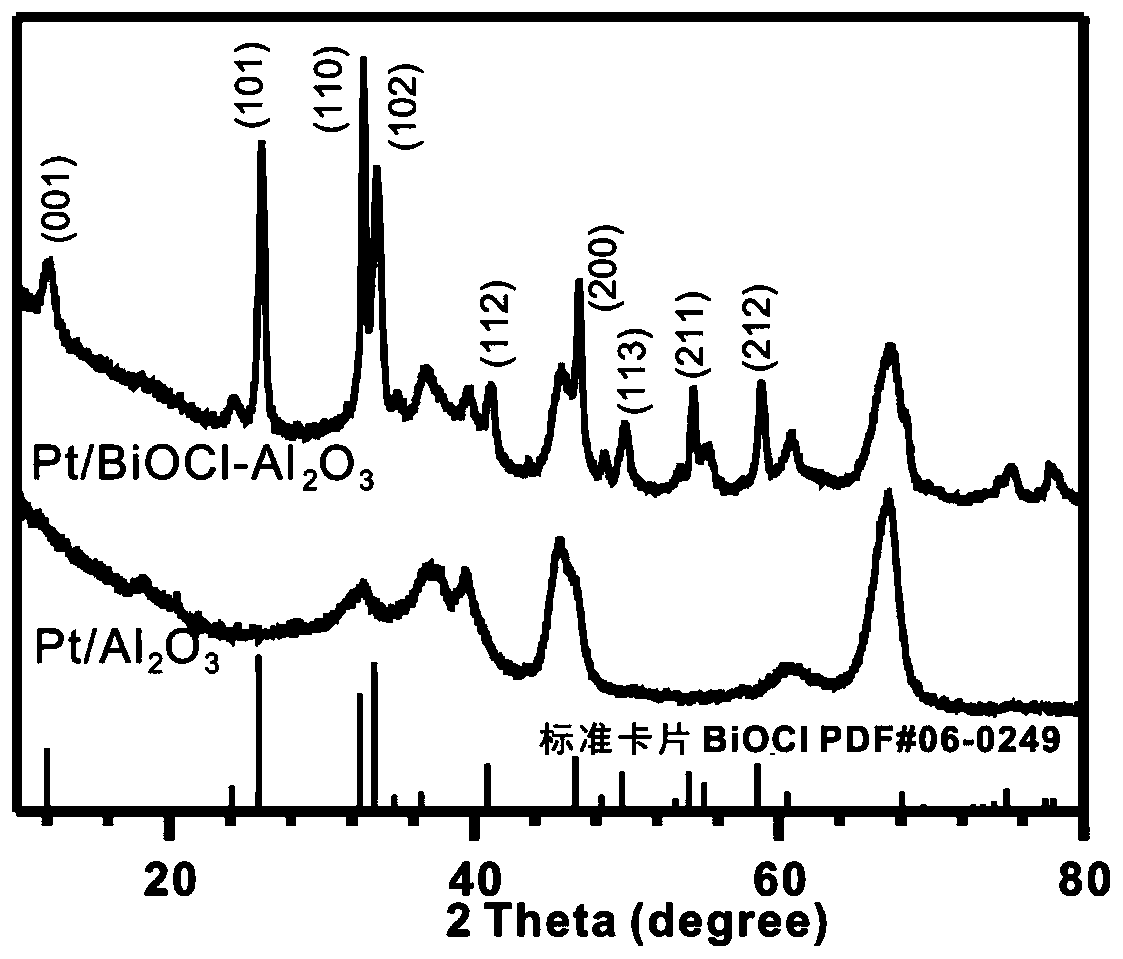
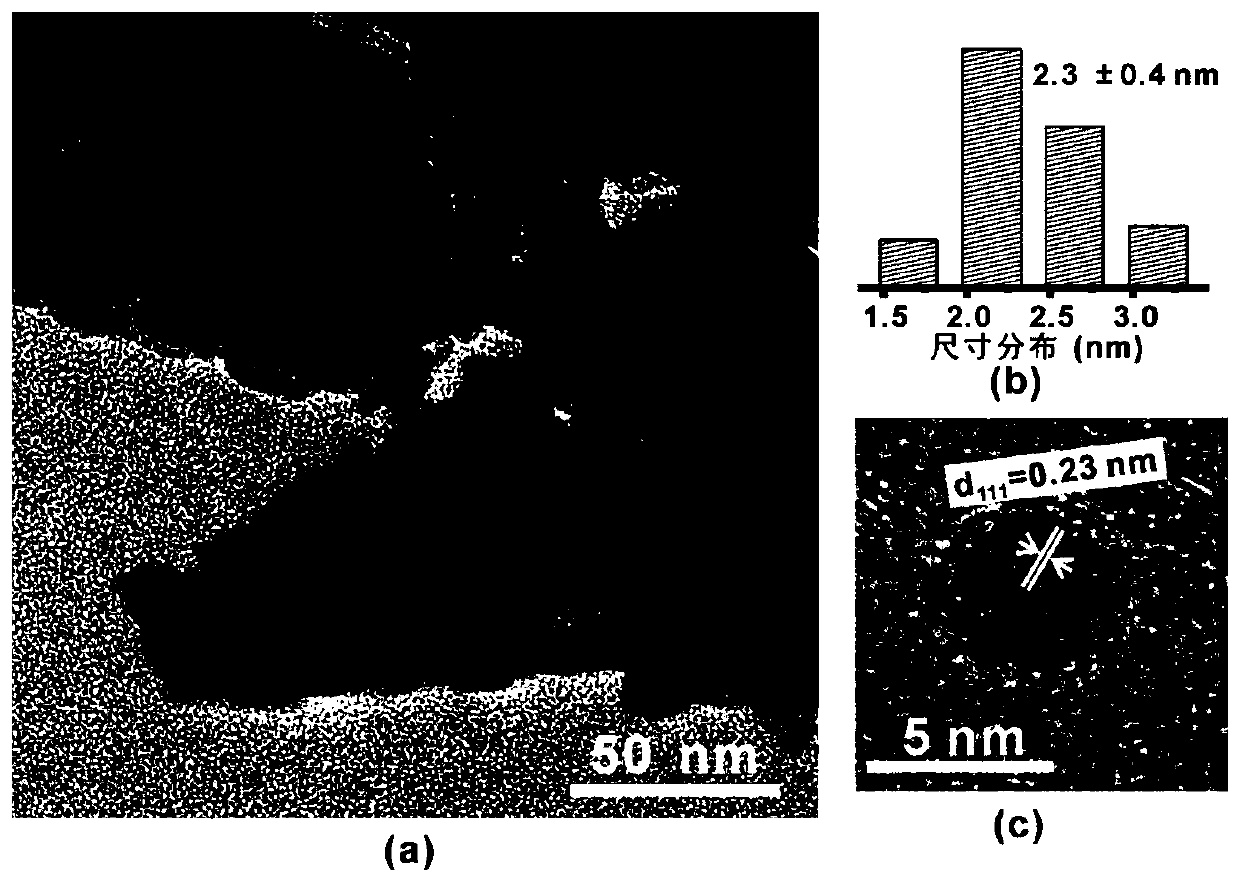
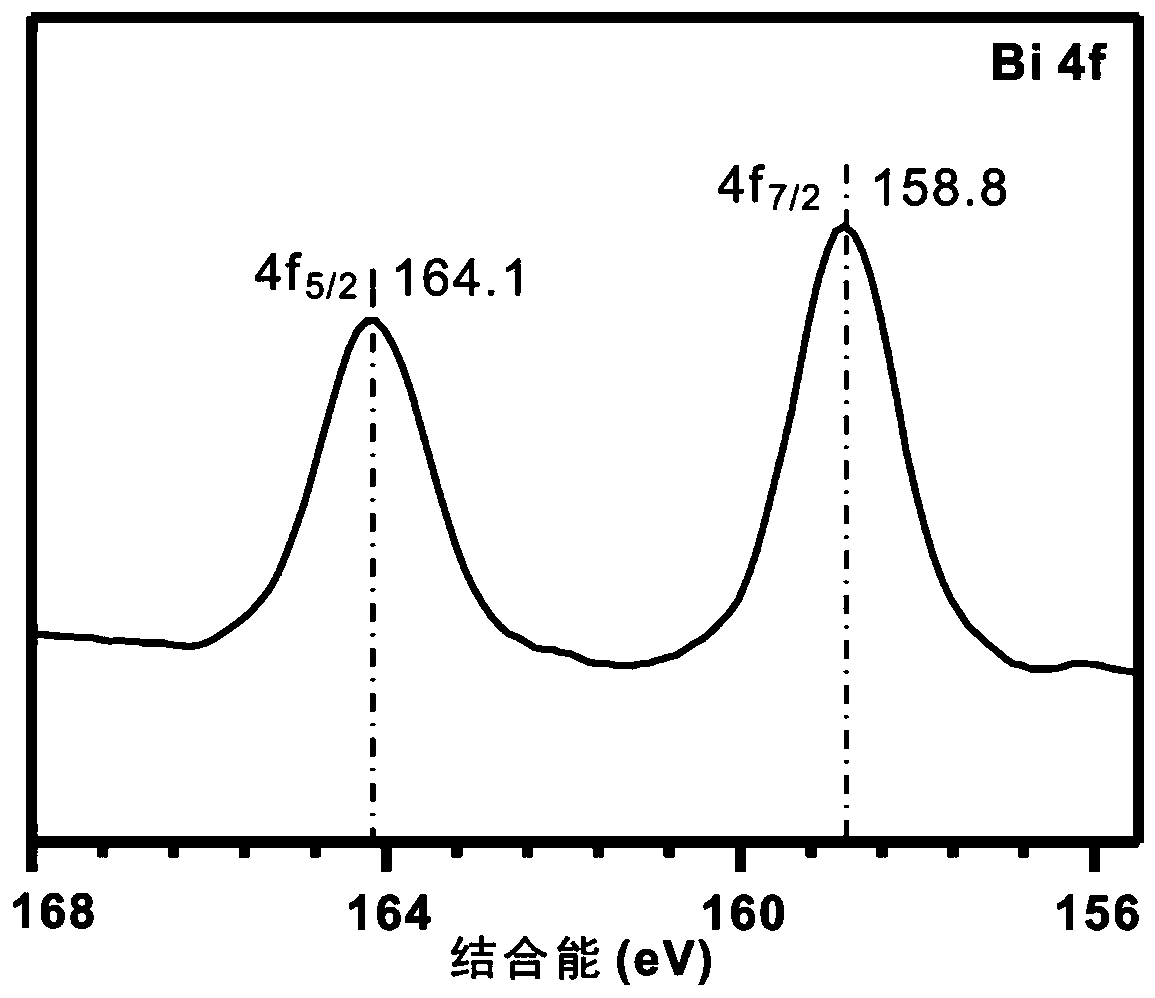
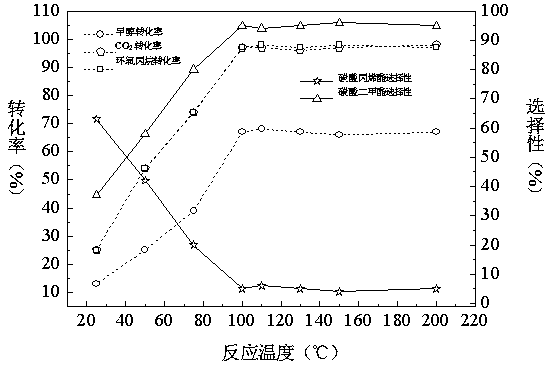
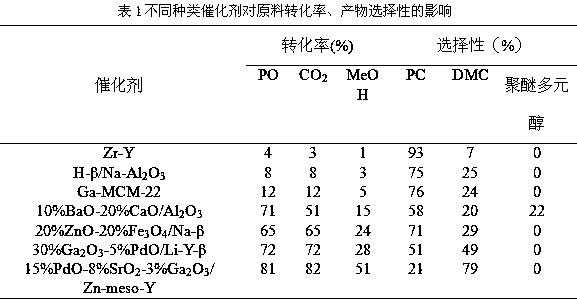
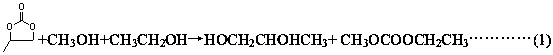Patents
Literature
49results about How to "Reaction process cleaning" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid carried ion liquid-nanometer metal particle catalyst, and its preparing method, and application in synthesis of arylamine
InactiveCN101045213AAvoid churnNot easy to eluteOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitro compoundMetal particle
A catalyst carrying ionic liquid and metallic nanoparticles for preparing high-quality arylamine by hydrocatalyzing aromatic nitro compound is prepared through preparing immobilized ionic liquid by bonding the ionic liquid onto carrier, binding it with the active component containing transition metal or precursor, and reductive exchange. Its advantages are low reaction temp, long service life and no waste generation.
Owner:SHAANXI NORMAL UNIV
Esterifiable modification method of lignocelluloses and esterifiable modified lignocelluloses
InactiveCN102311550AAdvantages of chemical modificationCharacteristic controlCelluloseEsterification reaction
The invention discloses an esterifiable modification method of lignocelluloses and esterifiable modified lignocelluloses. The esterifiable modification method comprises the following steps: dissolving lignocelluloses in an ionic liquid; and carrying out esterification reaction on the lignocelluloses and an esterifying agent. The method disclosed by the invention has the characteristics that the prices of the used raw materials are low, the technologic process is simple, the reaction process has no pollution, a reaction system can be reused, the hydroxyl substitution degree of a prepared lignocellulose esterification material can be controlled, and the like; and the method is one of important methods for industrial transformation and utilization of renewable lignocellulose resources.
Owner:BEIJING FORESTRY UNIVERSITY
Copper ion fluorescent detecting probe and method for making same and purpose
InactiveCN101261228AHigh fluorescence intensityRealize in situ detectionFluorescence/phosphorescenceViscous liquidFluorescence
A copper ion fluorescent probe and a preparation method and the use thereof relate to a fluorescence molecule probe, in particular to a copper ion fluorescence molecule probe and the preparation method and the application of the probe in the identification and detection of heavy metal. A copper ion fluorescence molecule probe with better effect which can test the heavy metallic copper ions in aqueous solution and the preparation method and the application are provided. Polyethyleneimine is allocated to be polyethyleneimine solution; the polyethyleneimine solution is taken out, filtered and poured into the lining of polytetrafluoroethylene which is sealed by a stainless steel cover; a solvent is distilled after reactions and the yellow viscous liquid-like copper ion fluorescent probe is gained. The copper ion fluorescent probe can be applied to the detection of the heavy metallic copper ions, in particular to the detection of the copper ions in biological tissues and environmental wastewater.
Owner:XIAMEN UNIV
Method for preparing benzaldehyde by catalytically oxidizing phenylcarbinol through Pt/BiOCl-metal oxide serving as catalyst
ActiveCN107042108AThe type of carrier can be controlledHigh selectivityCatalyst carriersOrganic compound preparationBenzaldehydeManganese
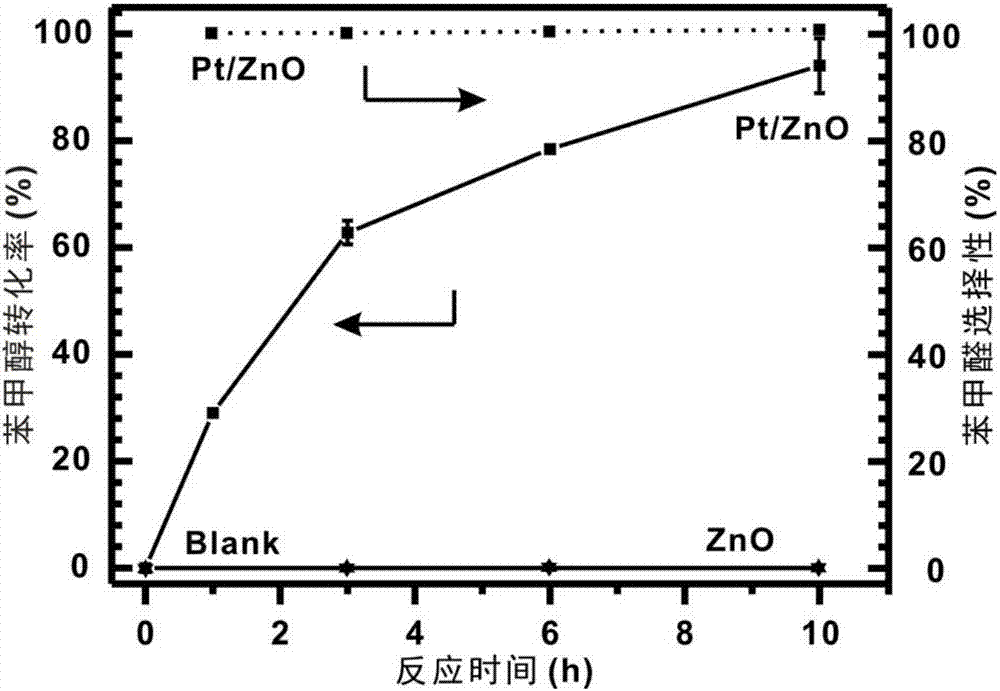
The invention discloses a method for preparing benzaldehyde by catalytically oxidizing phenylcarbinol through Pt / BiOCl-metal oxide serving as a catalyst. The method comprises the following steps: by taking a noble metal Pt / BiOCl-metal oxide compound system as the catalyst, dispersing phenylcarbinol and the catalyst into water, and continuously stirring under a relatively low-temperature dark condition for 3 to 10 hours, wherein the generation selectivity of the benzaldehyde can be 99 percent or above, and the yield of the benzaldehyde is retained within a range of 80 to 100 percent according to different types of carriers. The method has the following active effects that 1, the reaction can be carried out at relatively low temperature, such as room temperature of 25 DEG C; 2, low-cost air is used as an oxidant, so that the problem of environmental pollution caused by the fact that a high-cost metal such as chromium and manganese is used as the oxidant is solved; 3, no alkaline substances serving as an auxiliary catalyst are added into a reaction system; 4, basically no side effect is caused in a reaction process, and the generation selectivity of the benzaldehyde is retained at 99 percent or above; 5, equipment devices for reaction and an operation process are simple.
Owner:广西鲁桥新材料有限公司
Preparation method of N-methyl-4-methoxyaniline
ActiveCN105924363AImprove responseReaction process cleaningOrganic compound preparationAmino-hyroxy compound preparationSolventPetroleum
The invention relates to a method for preparing N-methyl-4-methoxyaniline, and relates to the field of petroleum additive and chemical pharmaceutical intermediates. According to the invention, the preparation of N-methyl-4-methoxyaniline is divided into two steps which are N-methyl-4-methoxyaniline synthesis and N-methyl-4-methoxyaniline rectification. According to the method, N-methyl-4-methoxyaniline is synthesized through catalytic hydrogenation under a condition with a solvent. During an N-methyl-4-methoxyaniline conversion synthesis process, the hydrogenation reaction is carried out with a catalyst, such that the process has the advantages of mild reaction, short time, low temperature, simple operation and the like. During the rectification process, first the organic solvent is removed, then rectification is carried out, such that a qualified N-methyl-4-methoxyaniline standard product can be obtained.
Owner:JIANGSU ZHONGDAN CHEM TECH
Method for synthesizing spherical superparamagnetic ferrite nano druse
InactiveCN101913852ANarrow particle size distributionGood dispersionNanostructure manufactureDivalent metalRoom temperature
The invention discloses a method for synthesizing a spherical superparamagnetic ferrite nano druse, relates to a ferrite nano druse and provides a method for directly synthesizing the spherical superparamagnetic ferrite nano druse without adding a protective agent. The method comprises the following steps of: preparing solution required by a spherical ferroferric oxide nano druse, namely dissolving ferric salt and urea in an ethylene glycol solvent to obtain solution A, wherein the molar ratio of the ferric salt to the urea is 1:(2-4); adding divalent metal salt into the solution A to obtain solution B, wherein the molar ratio of the divalent metal salt to the ferric salt is 2:(1-2); ultrasonically mixing the solution A and the solution B, putting the mixture into a polytetrafluoroethylene lining, and then putting the polytetrafluoroethylene lining into a steel kettle at constant temperature; and cooling the polytetrafluoroethylene lining at room temperature and washing black precipitates to obtain the spherical superparamagnetic ferrite nano druse.
Owner:XIAMEN UNIV
Method for preparing copper iodide P-type transparent semi-conductor thin film material at room temperature
ActiveCN108677155AImprove conductivityImprove permeabilityVacuum evaporation coatingSputtering coatingComposite filmOptical property
The invention provides a method for preparing copper iodide P-type transparent semi-conductor thin film material at room temperature and relates to a preparation method of the copper iodide P-type transparent semi-conductor thin film material. The method aims to overcome the defects of the high reaction temperature, complex equipment operation and long reaction time existing in an existing physical method universally, and solve the problem that the electrical property and the optical property of copper iodide thin films prepared through traditional copper film one-time iodization film formingare low. The method for preparing the copper iodide P-type transparent semi-conductor thin film material at the room temperature is completed through the steps of washing a target material and a substrate, preparing a Cu thin film, preparing a single-layer CuI thin film, and preparing a CuI composite thin film circularly.
Owner:HARBIN INST OF TECH
Electrochemical preparation method of uniform and compact cuprous iodide semiconductor film
InactiveCN101871111AImprove compactnessLow costElectrolytic inorganic material coatingSemiconductor devicesPlatinumIodide
The invention discloses an electrochemical preparation method of a uniform and compact cuprous iodide semiconductor film, which comprises the following steps: 1) washing ITO conductive glass or silicon wafers with acetone 2 to 3 times, cleaning the ITO conductive glass or the silicon wafers in an ultrasonic cleaning machine with deionized water, then activating the ITO conductive glass or the silicon wafers in 10% of nitric acid solution, and finally washing with deionized water repeatedly; 2) mixing 0.002-2mol / L of Cu (NO3) 2, 0.002-2mol / L of KI, 3.4 x 10<-6>-1.7 x 10<-3>mol / L of K30 polyvinylpyrrolidone, and adjusting the pH value to 1 to 5 with 2mol / L of HNO3 or 0.1mol / L of NaOH solution to obtain electrolyte; and 3) taking the ITO conductive glass or the silicon wafers as a working electrode, taking a platinum sheet electrode as a counter electrode, and taking a saturated calomel electrode as a reference electrode to obtain the compact cuprous iodide semiconductor film by eletrodeposition, wherein the eletrodeposition voltage of the relative saturated calomel electrode is 0.1-0.4V, the eletrodeposition time is 1-30 minutes, and the electrodeposition temperature is 25-80 DEG C. The invention has the advantages of simple equipment, low cost, low reaction condition requirements and the like.
Owner:ZHEJIANG UNIV
Method for preparing dimethyl succinate
InactiveCN104878048AMeet operational requirementsReaction process greenOrganic compound preparationCarboxylic acid esters preparationChemical industryDisodium Succinate
The invention relates to a method for preparing dimethyl succinate. The method comprises the following steps: (1) preparing disodium succinate fermented liquid; (2) treating the disodium succinate fermented liquid; and (3) preparing dimethyl succinate by using a bipolar-membrane electrodialysis apparatus. The processes such as catalyst cleaning, regeneration, and the removal of a deactivated catalyst, and the like existing in the traditional preparation process are omitted; and the whole reaction process is green and clean, and completely conforms to the operating requirements of the modern chemical industry.
Owner:镇江博睿兴邦生物科技有限公司
Method for efficiently preparing benzaldehyde by using air as oxidation agent to catalyze benzyl alcohol in alkali-free water phase system at room temperature
ActiveCN107042107AEfficient conversionMild reaction conditionsCatalyst carriersOrganic compound preparationBenzaldehydeBENZYL ALCOHOL/WATER
The invention discloses a method for efficiently preparing benzaldehyde by using air as an oxidation agent to catalyze benzyl alcohol in an alkali-free water phase system at room temperature. According to the method, a precious metal Pt / metal oxide (zinc oxide, titanium oxide or reduced bismuth oxide) composite system is used as a catalyst, and the benzyl alcohol and the catalyst are dispersed into water and are then continuously stirred for 5-10h at low temperature in a dark place; the generation selectivity of the benzaldehyde can reach 99% or above, and the yield of the benzaldehyde is maintained within a range of 75-100% with the different types of carriers. The method has the positive effects that 1, a reaction can be carried out at the lower temperature such as room temperature 25 DEG C; 2, the cheap air is taken as the oxidation agent, so that the problem of environmental pollution caused by high valence metal such as chromium and manganese which are used as oxidation agents can be solved; 3, alkaline substances are not added into the reaction system as catalyst promoters; 4, the reaction process is basically free from a side reaction, and the generation selectivity of the benzaldehyde is maintained to be 99% or above; 5, equipment and operation technology of the reaction are simple.
Owner:浙江知多多网络科技有限公司
Novel process for synthesizing cyclohexyl formic acid by benzoic acid hydrogenation
InactiveCN1749234AMild reaction conditionsHigh purityCarboxylic preparation by ozone oxidationMetal/metal-oxides/metal-hydroxide catalystsBenzoic acidHydrogen
The novel process of hydrogenating benzoic acid to synthesize cyclohexyl formic acid includes the catalytic cyclization reaction of benzoic acid via contact with hydrogen inside supercritical CO2 and cyclohexyl formic acid without solvent in the presence of metal rhodium catalyst and under mild reaction condition; and subsequent extraction to separate product from the catalyst by means of the dissolubility of cyclohexyl formic acid in supercritical CO2. The technological process of the present invention has the advantages of mild reaction condition, clean reaction process, no side product and waste, no need of post-separation, and high product yield and quality.
Owner:JIANGSU QINGQUAN CHEM CO LTD
Process for one-step synthesis of methyl ethyl carbonate and co-production of ethylene glycol by using ethylene oxide
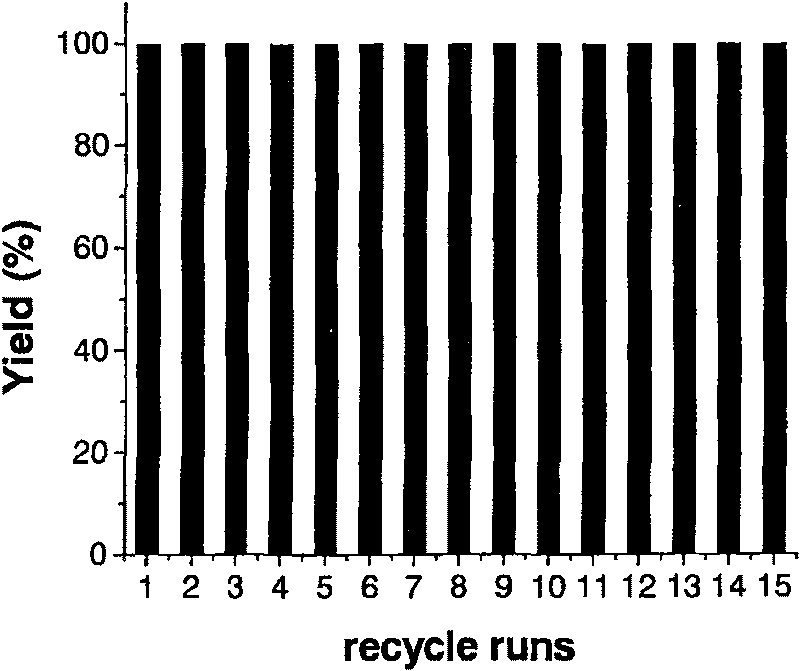
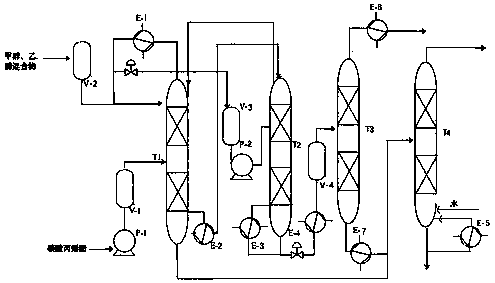
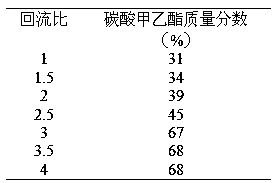
InactiveCN108129310AShort synthetic routeReaction process cleaningProductsOrganic compound preparationMethyl carbonateEthylene oxide
The invention discloses a process for one-step synthesis of methyl ethyl carbonate and co-production of ethylene glycol by using ethylene oxide, and relates to the process of synthesizing the methyl ethyl carbonate. According to the process, azeotropic separation is carried out while reaction rectification is carried out, and extraction and separation are carried out on a product without a solvent, so that the technological process of a conventional synthesis process of the methyl ethyl carbonate is greatly simplified, meanwhile multiple components can be separated at one time, and the separation purity is high. The selectivity of the methyl ethyl carbonate in the crude product obtained by the reaction can be up to 70%. The by-products, dimethyl carbonate and diethyl carbonate, can be directly separated out as products, and can be recycled for continued reaction for generating the methyl ethyl carbonate; and the by-product ethylene glycol can be separated out through simple distillation as a bulk raw material. The whole reaction process is clean, efficient and free of pollution, and no waste is generated. The optimal temperature for separating ethylene glycol by using a T4 ethyleneglycol batch rectifying tower is 140-155 DEG C, and the ethylene glycol content can reach 96% or above under the condition.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for synthesizing para aminophenylmethylether by catalytic hydrogenation of paranitroanisole
InactiveCN101798272AEasy to separateImprove protectionOrganic compound preparationBulk chemical productionP-nitroanisoleHydrazine compound
The invention provides a method for synthesizing para aminophenylmethylether by catalytic hydrogenation paranitroanisole. In the method, the paranitroanisole is subjected to catalytic reduction reaction with hydrogen to generate the para aminophenylmethylether in the presence of a load type palladium catalyst in a supercritical carbon dioxide reaction medium under mild conditions. Compared with the traditional processes, such as a nitrobenzene method, a sodium sulfide or ferrum reduction method, a method for synthesizing the para aminophenylmethylether by reducing the paranitroanisole by activated carbon load type palladium catalyst hydrazine hydrate, and the like, the invention has the advantages of mild reaction condition, fast reaction, clean reaction process and easy product separation, achieving the product yield more than 99 percent, and the like; in addition, in the invention, reaction temperature is reduced from 75-125 DEG C in the nitrobenzene method to 50-80 DEG C, and reaction time is reduced from 130 minutes in the method for synthesizing the para aminophenylmethylether by reducing the paranitroanisole by the activated carbon load type palladium catalyst hydrazine hydrate to 10-30 minutes without adding any organic solvent and additive or generating any side products in the reaction process.
Owner:CHANGCHUN UNIV OF TECH
Method for synthesizing carbonic acid symmetric ester in one step with ethylene carbonate and alcohols
InactiveCN108047040AEfficient responseShort synthetic routeMolecular sieve catalystsOrganic compound preparationPolyolAlcohol
The invention relates to a method for synthesizing carbonic acid symmetric ester in one step with ethylene carbonate and alcohols, in particular to a method for synthesizing the carbonic acid symmetric ester. A high-efficiency alkaline catalyst of a composite pore structure is used for preparing synthesizing the carbonic acid symmetric ester in one step with ethylene carbonate and various alcoholssuch as ROH and diols, ethylene glycol, diethylene glycol, polyol and the like. A crude product obtained through a reaction only contains the carbonic acid symmetric ester and ethylene glycol. The conversion rate of the ethylene carbonate can reach 95% at most. The byproduct ethylene glycol serving as a bulk raw material can be separated through simple distillation. The whole reaction process isclean, efficient, free of pollution and free of waste. The molar ratio of the ethylene carbonate to various alcohols is 1:3, the reaction pressure is 5 MPa, the reaction temperature is 100 DEG C, theair speed is 5 h<-1>, and after being used for 5000 h, the catalyst is still active and good in stability.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for synthesizing symmetrical compound of aroma urea group
InactiveCN1626510AEasy to separateReaction process cleaningUrea derivatives preparationOrganic compound preparationBiotechnologyNitro compound
A process for synthesizing the symmetric arylurea compound features that the aromatic nitro compound takes part in carbonylation reaction in organic solvent under existance of CO, Se powder as catalyst and alkali as cocatalyst to obtani target produst. The Se powder is then oxidized by O2 or air for separating it out and reusing it.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
4-methyl-2-nitroaniline synthesis method
InactiveCN107759479AMild reaction conditionsNo need for high temperatureCarbamic acid derivatives preparationOrganic compound preparationNitrosoMethylaniline
The invention discloses a 4-methyl-2-nitroaniline synthesis method, which comprises: carrying out amino protection with ethyl chloroformate by using 4-methylaniline as a raw material to generate N-(p-toluene)ethyl carbamate; adding an oxidant and a copper salt catalyst, and carrying out a reaction for a certain time at a temperature of 50-120 DEG C in a reaction solvent by using a nitroso-containing compound as a nitrating agent to prepare a corresponding protected o-nitro-p-toluidine; and carrying out hydrolysis to obtain the target product. According to the present invention, the method hasadvantages of simple preparation process, mild reaction condition, high yield and environment protection.
Owner:NANJING UNIV OF SCI & TECH
Method for synthesis of symmetrical carbonate and co-production of 1,2-propylene glycol
InactiveCN108129313AEfficient responseReaction process cleaningMolecular sieve catalystsOrganic compound preparationDistillationReaction temperature
The invention discloses a method for synthesis of symmetrical carbonate and co-production of 1,2-propylene glycol, and relates to the method for synthesizing the symmetrical carbonate. According to the method provided by the invention, a high-efficiency basic catalyst with a composite pore structure is used for one-step synthesis of the symmetrical carbonate by using propylene carbonate and various alcohols (ROH, wherein the R can be selected from various alcohols such as straight-chain alcohols, iso-alcohols, aromatic alcohols, phenols, diols such as ethylene glycol and diethylene glycol, polyols, and the like). The obtained crude product obtained by a reaction only contains the symmetrical carbonate and the 1,2-propylene glycol, wherein the conversion rate of the propylene carbonate canbe up to 95%. The by-product 1,2-propylene glycol can be separated out through simple distillation as a bulk raw material. The whole reaction process is clean, efficient and free of pollution, and nowaste is generated. When the molar ratio of the propylene carbonate to the various alcohols is 1:3, the reaction pressure is 5 MPa, the reaction temperature is 100 DEG C, and the space velocity is 5 h<-1>, the catalyst is not deactivated and has good stability after being used for 5000 hours.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Novel process for synthesizing cyclohexyl formic acid by benzoic acid hydrogenation
InactiveCN100352797CMild reaction conditionsHigh purityCarboxylic preparation by ozone oxidationMetal/metal-oxides/metal-hydroxide catalystsBenzoic acidPtru catalyst
Owner:JIANGSU QINGQUAN CHEM CO LTD
Method for synthesizing ethyl methyl carbonate in one-step and co-producing ethylene glycol from ethylene oxide
InactiveCN108191661AEfficient responseReaction process cleaningProductsMolecular sieve catalystsCarbon dioxideDistillation
The invention discloses a method for synthesizing ethyl methyl carbonate in one step and co-producing ethylene glycol from ethylene oxide, and relates to methods for synthesizing ethyl methyl carbonate. The method is characterized in that a high-efficiency alkaline catalyst with a composite pore structure is used for one-step synthesis of ethyl methyl carbonate from ethylene oxide, carbon dioxide,methanol and ethanol. The obtained crude product of the reation contains dimethyl carbonate, ethyl methyl carbonate, diethyl carbonate and ethylene glycol, wherein the selectivity of methyl ethyl carbonate can reach up to 70% at maximum, byproducts of dimethyl carbonate and diethyl carbonate can be directly isolated as products, or can be recycled to react continuously to form ethyl methyl carbonate, and the byproduct ethylene glycol as a bulk raw material can be separated through simple distillation. The entire reaction process is clean, efficient, non-polluting and free of generating any waste. When the molar ratio of ethylene oxide: carbon dioxide: methanol: ethanol is 1:1:3:2, the reaction pressure is 5 MPa, the reaction temperature is 100 DEG C, and the space velocity is 5 h<-1>, thecatalyst does not lose activity after being used for 5000 h, and the stability is better.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Process for synthesizing ethyl methyl carbonate in one step and co-producing ethylene glycol
InactiveCN108191605ASimple processHigh separation purityOxygen-containing compound preparationOrganic compound preparationReactive distillationSolvent
The invention discloses a process for synthesizing ethyl methyl carbonate in one step and co-producing ethylene glycol, and relates to manufacturing processes of preparing ethyl methyl carbonate. Theprocess is characterized in that ethyl methyl carbonate, diethyl carbonate and ethylene glycol are directly synthesized in one step under the catalysis of a multifunctional composite alkaline materialcatalyst; when reactive distillation is carried out, azeotropic separation is carried out simultaneously, and no solvent is needed to perform extraction separation on the products, so that technicalprocess of conventional ethyl methyl carbonate synthesis is greatly simplified, meanwhile, multiple components can be separated at one time, and the separation purity is high. The selectivity of methyl ethyl carbonate in the obtained crude product of the reaction can reach up to 70% at maximum, byproducts of dimethyl carbonate and diethyl carbonate can be directly isolated as products, or can be recycled to react continuously to form ethyl methyl carbonate, and the byproduct ethylene glycol as a bulk raw material can be separated through simple distillation. The entire reaction process is clean, efficient, non-polluting and free of generating any waste.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for one-step synthesis of ethyl methyl carbonate from propylene oxide and co-production of 1,2-propanediol
InactiveCN108164418AEfficient responseReaction process cleaningProductsReagentsDistillationMethyl carbonate
The invention relates to a method for synthesizing ethyl methyl carbonate, and concretely relates to a method for one-step synthesis of ethyl methyl carbonate from propylene oxide and co-production of1,2-propanediol. An efficient alkaline catalyst having a compopsite pore structure is used in the one-step reaction of the propylene oxide, carbon dioxide, methanol and ethanol for synthesizing the ethyl methyl carbonate. A crude product obtained through the reaction contains dimethyl carbonate, ethyl methyl carbonate, diethyl carbonate and 1,2-propanediol, wherein the ethyl methyl carbonate selectivity reaches up to 70%. Byproducts dimethyl carbonate and diethyl carbonate can be directly separated as products, also can be recycled, and are continuously reacted to generate the ethyl methyl carbonate, and the product 1,2-propanediol can be separated through simple distillation as a popular raw material. The method has the advantages of cleanness, high efficiency, no pollution and no wastesin the whole reaction process. The catalyst is not inactive after being used at a molar ratio of the propylene oxide to the carbon dioxide to the methanol to the ethanol of 1:1:3:2 under a reaction pressure of 5 MPa at a reaction temperature of 100 DEG C under an air speed of 5 h<-1> for 5000 h, and has a good stability.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Pt/biocl-metal oxide is used as catalyst to prepare the method of benzaldehyde by catalytic oxidation of benzyl alcohol
ActiveCN107042108BThe type of carrier can be controlledHigh selectivityCatalyst carriersOrganic compound preparationSide effectBenzaldehyde
The invention discloses a method for preparing benzaldehyde by catalytically oxidizing phenylcarbinol through Pt / BiOCl-metal oxide serving as a catalyst. The method comprises the following steps: by taking a noble metal Pt / BiOCl-metal oxide compound system as the catalyst, dispersing phenylcarbinol and the catalyst into water, and continuously stirring under a relatively low-temperature dark condition for 3 to 10 hours, wherein the generation selectivity of the benzaldehyde can be 99 percent or above, and the yield of the benzaldehyde is retained within a range of 80 to 100 percent according to different types of carriers. The method has the following active effects that 1, the reaction can be carried out at relatively low temperature, such as room temperature of 25 DEG C; 2, low-cost air is used as an oxidant, so that the problem of environmental pollution caused by the fact that a high-cost metal such as chromium and manganese is used as the oxidant is solved; 3, no alkaline substances serving as an auxiliary catalyst are added into a reaction system; 4, basically no side effect is caused in a reaction process, and the generation selectivity of the benzaldehyde is retained at 99 percent or above; 5, equipment devices for reaction and an operation process are simple.
Owner:广西鲁桥新材料有限公司
Method for one-step synthesis of carbonic acid symmetric ester and co-production of 1,2-propanediol through propylene oxide
InactiveCN108586244AEfficient responseReaction process cleaningOxygen-containing compound preparationProductsDistillationReaction temperature
The invention relates to a method for one-step synthesis of a carbonic acid symmetric ester and co-production of 1,2-propanediol through propylene oxide, and relates to a method for synthesizing a carbonic acid symmetric ester. According to the present invention, the method is used for one-step synthesis of carbonic acid symmetric esters through propylene oxide, carbon dioxide and various alcohols(ROH can be a linear alcohol, an isomeric alcohol, an aromatic alcohol, a phenol, a diol such as 1,2-propylene glycol and diethylene glycol, polyol and various alcohols), the crude products obtainedthrough the reaction contain only the carbonic acid symmetric ester and the 1,2-propanediol, the selectivity of the carbonic acid symmetric ester can be up to 95%, and the by-product 1,2-propanediol as the bulk material can be separated through simple distillation, such that the whole reaction process is clean, efficient and non-polluting, and does not produce any waste; and the catalyst is not deactivated for 5000 h at the reaction temperature of 100 DEG C under the reaction pressure of 5 MPa at the space velocity of 5 h<-1> according to the molar ratio of propylene oxide to carbon dioxide tovarious alcohols of 1:1:3 so as to achieve good stability.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Preparation method and application of composition of 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetate
InactiveCN108419800AReaction process cleaningReduce hydrolysisPlant growth regulatorsBiocide2,4-Dichlorophenoxyacetic acidDrug effect
The invention provides a preparation method for a composition of 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetate. The preparation method comprises the following steps: reacting halogenated acetate with 2,4-dichlorophenate so as to obtain 2,4-dichlorophenoxyacetate; and directionally hydrolyzing 2,4-dichlorophenoxyacetate prepared in the previous step to obtain the composition of 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetate. According to the invention, a process route is adjusted and creative improvement is made from the foundation; halogenated acetate reacts with2,4-dichlorophenate so as to prepare 2,4-dichlorophenoxyacetate, and directional hydrolysis is carried out so as to obtain the composition of 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetate. The composition of 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetate of the invention has excellent quality, high stability and good drug effect.
Owner:SHANDONG RUNBO BIOTECH CO LTD
Method of one-step synthesis of asymmetric carbonate and co-production of 1,2-propylene glycol by using propylene oxide
InactiveCN108129262AOvercome expensiveEfficient responseProductsMolecular sieve catalystsChemistryPropylene glycol
The invention discloses a method of one-step synthesis of asymmetric carbonate and co-production of 1,2-propylene glycol by using propylene oxide, and relates to the method for synthesizing the asymmetric carbonate. A high-efficiency neutral catalyst with a composite pore structure is used for the one-step synthesis of the asymmetric carbonate by using the propylene oxide, carbon dioxide, methanoland various alcohols. The selectivity of the asymmetric carbonate in the crude product obtained by a reaction can be up to 70%. The by-products, dimethyl carbonate and dialkyl carbonates can be directly separated out as products, and can be recycled for continued reaction for generating the asymmetric carbonate; and the by-product 1,2-propylene glycol can be separated out through simple distillation as a bulk raw material. When the molar ratio of the propylene oxide to the carbon dioxide to the methanol to the other alcohols is 1:1:3:2, the reaction pressure is 5 MPa, the reaction temperatureis 100 DEG C, and the space velocity is 5 hours<-1>, the catalyst is not deactivated and has good stability after being used for 5000 hours. The whole reaction process is clean, efficient and free ofpollution, and no waste is generated.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Solid carried ion liquid-nanometer metal particle catalyst, and its preparing method, and application in synthesis of arylamine
InactiveCN101045213BAvoid churnNot easy to eluteOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitro compoundMetal particle
A catalyst carrying ionic liquid and metallic nanoparticles for preparing high-quality arylamine by hydrocatalyzing aromatic nitro compound is prepared through preparing immobilized ionic liquid by bonding the ionic liquid onto carrier, binding it with the active component containing transition metal or precursor, and reductive exchange. Its advantages are low reaction temp, long service life andno waste generation.
Owner:SHAANXI NORMAL UNIV
One-step technology for synthesis of ethyl methyl carbonate and co-production of 1,2-propanediol
InactiveCN108017540AShort synthetic routeReaction process cleaningOrganic compound preparationChemical industrySolventMultiple component
The invention provides a one-step technology for synthesis of ethyl methyl carbonate and co-production of 1,2-propanediol and relates to a technology for synthesizing ethyl methyl carbonate. Accordingto the technology, ethyl methyl carbonate, diethyl carbonate and 1,2-propanediol are directly synthesized in one step by catalysis of a multifunctional composite alkaline material catalyst. Azeotropic separation is performed synchronously while reactive distillation is performed, a solvent is not needed for extraction and separation of a product, and accordingly, the conventional technological process of synthesis of ethyl methyl carbonate is greatly simplified; meanwhile, multiple components are separated at a time, and the separation purity is high. The maximum selectivity of ethyl methyl carbonate in a crude product obtained by the reaction can reach 70%, and dimethyl carbonate and diethyl carbonate as byproducts can be taken as products to be separated directly and can also be recycled to be reacted continuously for generation of ethyl methyl carbonate, and the byproduct 1,2-propanediol can be taken as a bulk raw material to be separated by simple distillation. The whole reactionprocess is clean, efficient and pollution-free, and no waste is generated.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
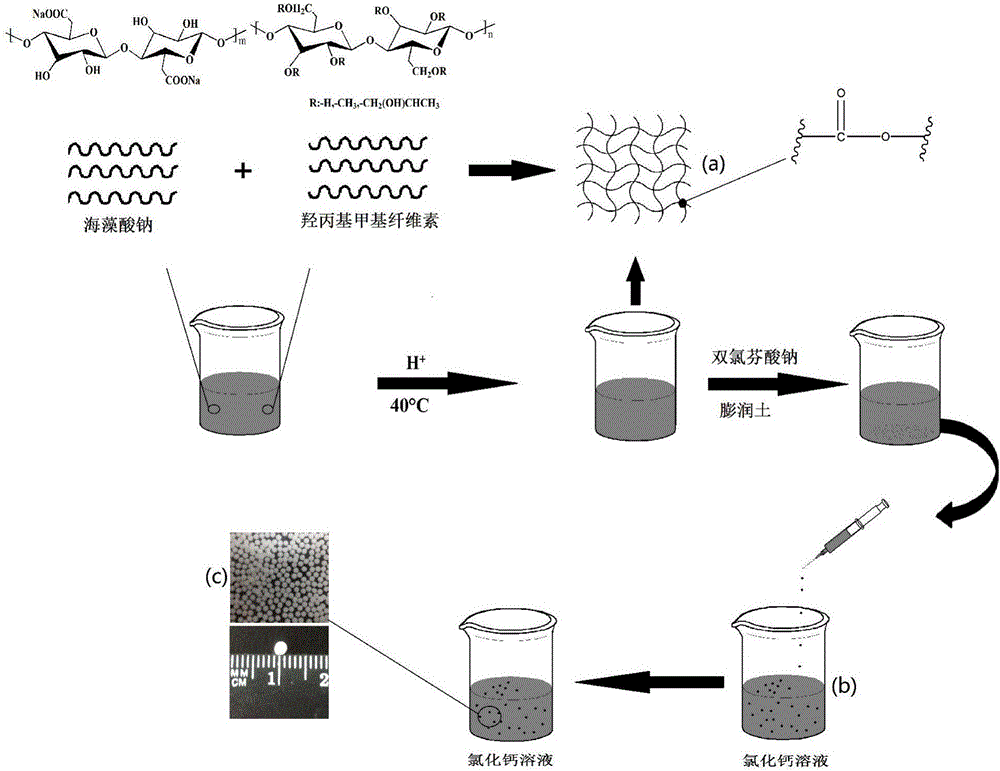
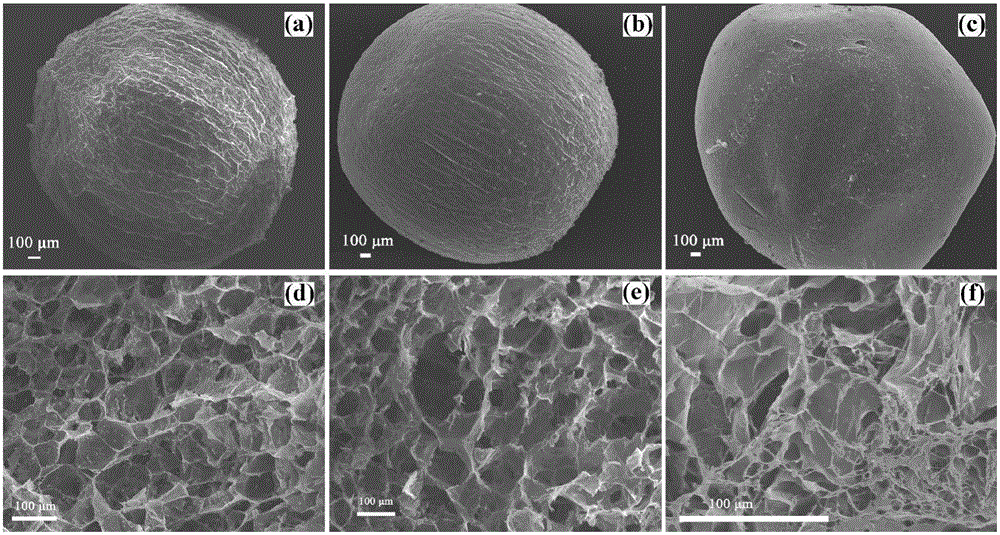
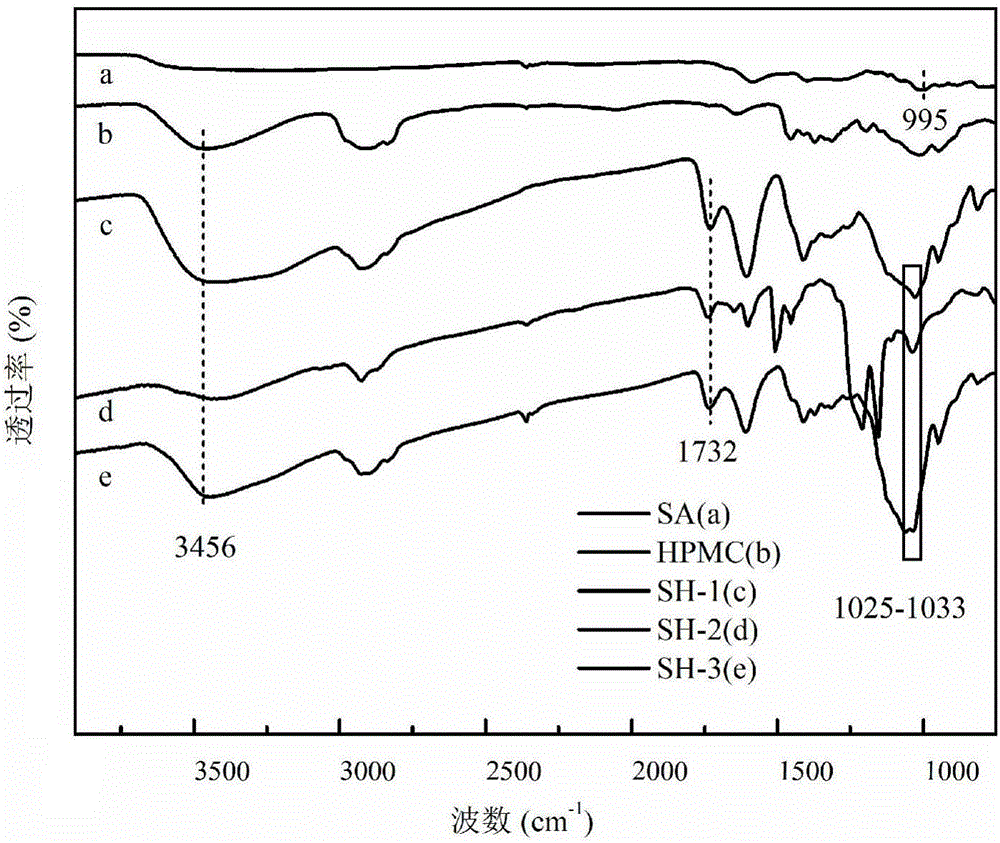
Preparation method of pH-sensitive type drug microspheres and pH-sensitive type drug microspheres prepared by preparation method
ActiveCN105963260AGood ball formingImprove stabilityOrganic active ingredientsAntipyreticMicrosphereCurative effect
The invention relates to a method for preparing sodium alginate (SA) / hydroxypropyl methylcellulose (HPMC) / bentonite pH-sensitive type drug microspheres. The method includes: sodium alginate and hydroxypropyl methylcellulose are polymerized for modification and then combined with diclofenac to prepare the pH-sensitive type drug microspheres. The pH-sensitive type drug microspheres prepared by the method have the advantages that the pH-sensitive type drug microspheres are good in spheronization and have evident selectivity to drug release media, the accumulation release amount of the pH-sensitive type drug microspheres is less than 1% especially in an acid environment, and the pH-sensitive type drug microspheres can protect drugs for treating intestinal diseases from being absorbed by the stomach or destroyed by gastric enzyme, prolong curative effect and increase drug stability and has promising application prospect and advantages in fields of pharmaceutical engineering and bio-based materials.
Owner:BEIJING FORESTRY UNIVERSITY
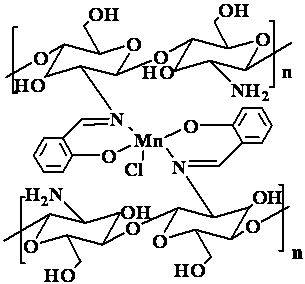
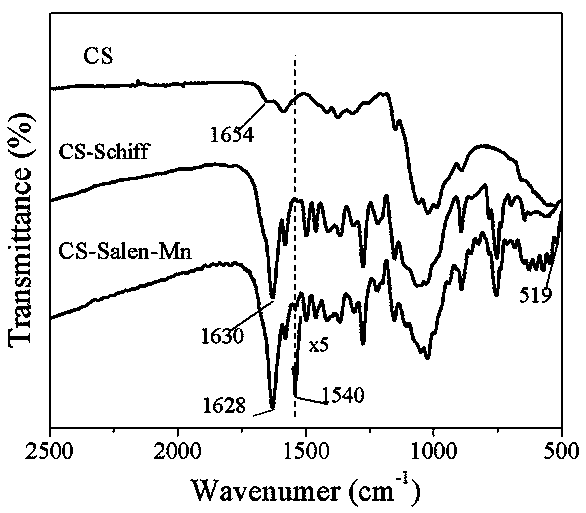
CS-Salen-Mn-type composite catalyst based on natural polymer chitosan and preparation method
InactiveCN109453809AGood conversion rate of oxidation reactionHigh selectivityOrganic chemistryOrganic compound preparationChemical industryMANGANESE ACETATE
The invention discloses a composite catalyst which is prepared by using an amino rich on the surface of natural polymer chitosan (CS for short) to be condensed with a formyl group in salicylaldehyde so as to form an imine ligand, and coordinating with manganese acetate and a preparation method, and belongs to the field of chemical industries. The composite catalyst is named as: CS-Salen-Mn, and astructural formula of the CS-Salen-Mn-type composite catalyst is as shown in Figure 1, wherein CS is used as a parent; the salicylaldehyde is a ligand; acetic acid is an organic solvent; Mn(OAc)2.4H2Ois used as a coordination center; an Mn atom in the CS-Salen-Mn is directly connected with an N atom forming the imine ligand in the chitosan by a coordination bond. The CS-Salen-Mn-type composite catalyst based on the natural polymer chitosan is designed and prepared by using a condensation and coordination technology. The composite catalyst CS-Salen-Mn has the advantages of simple preparation process, good reaction specificity, and high methyl phenyl sulfide oxidation reaction selectivity, can be recycled repeatedly and is pollution-free in a reaction process.
Owner:CHANGCHUN UNIV OF TECH
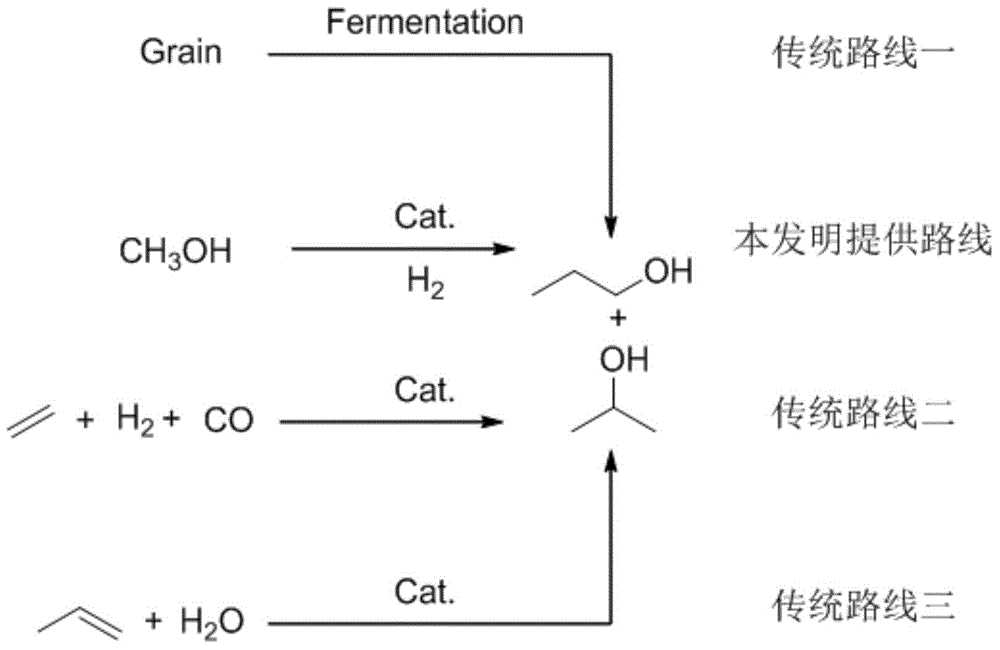
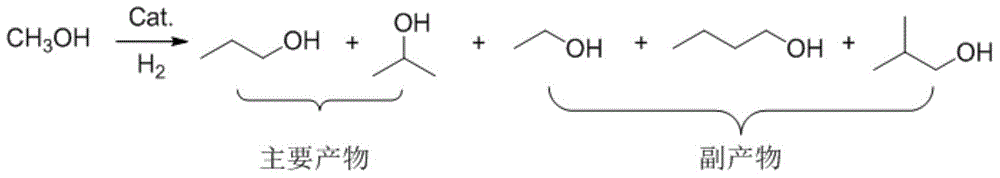
Method for preparing propanol through catalytic conversion of methanol
InactiveCN104892361AExpand downstream useReduce dependenceMolecular sieve catalystsOrganic compound preparationChemical industryFixed bed
The invention relates to a method for preparing propanol through catalytic conversion of methanol. According to the method, methanol is directly used as a raw material, a molecular sieve supporting composite metal oxide containing two or more than two elements selected from iron, cobalt, manganese, copper, molybdenum, vanadium, tungsten and chromium is adopted as a catalyst, and in a fixed bed reactor, methanol is subjected to catalytic conversion to prepare the propanol. According to the present invention, the method is the propanol preparing route directly adopting the methanol as the raw material, and directly adopts the domestic excess methanol as the raw material to prepare the important chemical industry raw material propanol so as to expand the downstream use of the methanol, and the catalyst is the non-noble metal catalyst and is cheap and easily-available, such that the method has important application prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com