Method for synthesizing spherical superparamagnetic ferrite nano druse
A technology of superparamagnetic magnets and synthesis methods, which is applied in nanotechnology, nanotechnology, nanostructure manufacturing, etc., can solve problems such as application limitations, and achieve the effects of uniform shape, stable properties, and narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Measure 16ml of ethylene glycol solution.
[0030] 2) Dissolve 404 mg of ferric nitrate nonahydrate and 120 mg of urea in the solution of step 1), and sonicate to make the solution uniform.
[0031] 3) Transfer the solution obtained in 2) into a polytetrafluoroethylene liner with a volume of 18 ml.
[0032] 4) Put the inner lining in the steel kettle, screw it tightly and put it into the oven. 180℃ constant temperature for 12h.
[0033] 5) After the reaction is completed, the reaction kettle is cooled down to room temperature with the oven, the solution in the polytetrafluoroethylene lining is poured out, and centrifuged to obtain a solid product, which is washed several times with ethanol and water in turn to obtain the target product.
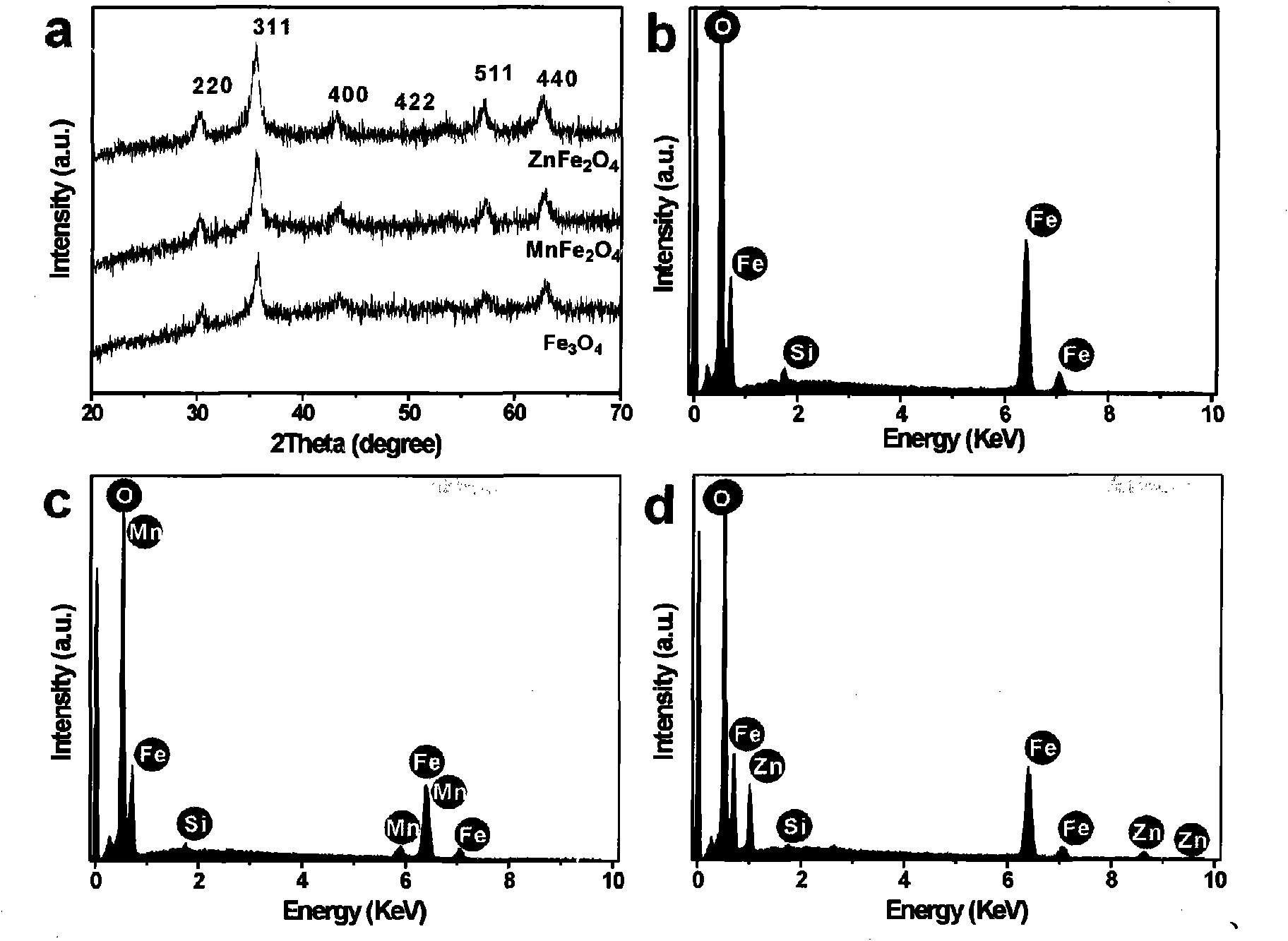
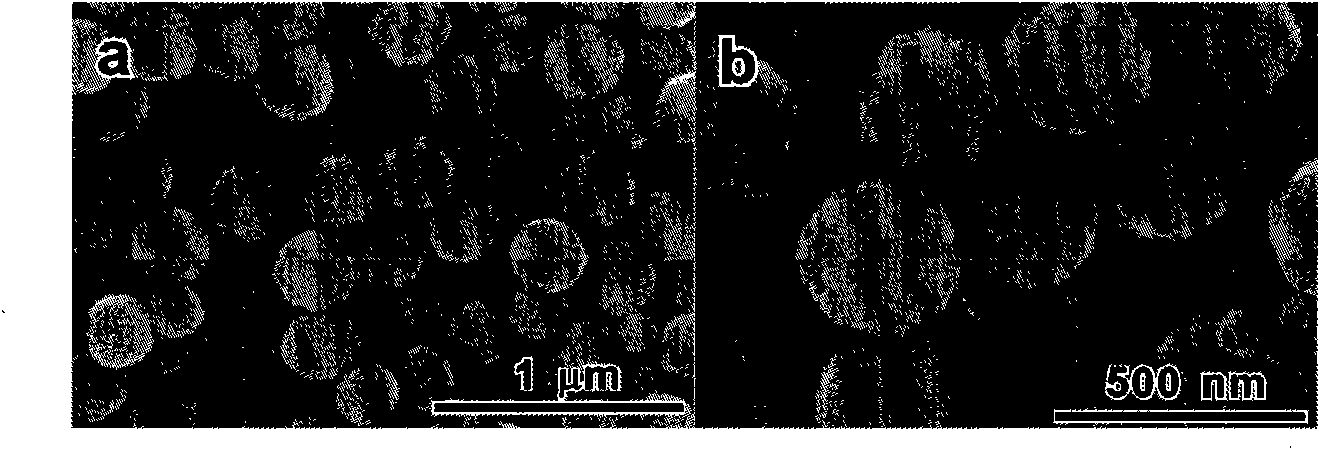
[0034] Depend on figure 2 It can be seen that the spherical ferric oxide nano-clusters have a uniform morphology, a particle size of about 220 nm, and a primary particle size of about 10 nm. Depend on figure 1 (a) It can be se...
Embodiment 2
[0036] 1) Measure 16ml of ethylene glycol solution.
[0037] 2) Dissolve 404 mg of ferric nitrate nonahydrate, 68 mg of zinc chloride and 120 mg of urea in the solution of step 1), and ultrasonically make the solution uniform.
[0038] 3) Transfer the solution obtained in 2) into a polytetrafluoroethylene liner with a volume of 18 ml.
[0039] 4) Put the inner lining in the steel kettle, screw it tightly and put it into the oven. Constant temperature at 200°C for 12h.
[0040]5) After the reaction is completed, the reaction kettle is cooled down to room temperature with the oven, the solution in the polytetrafluoroethylene lining is poured out, and centrifuged to obtain a solid product, which is washed several times with ethanol and water in turn to obtain the target product.
[0041] Depend on image 3 It can be seen that the particle size of the obtained spherical zinc ferrite nanoclusters is about 350 nm, and the size of the primary particles is about 11 nm. Depend on ...
Embodiment 3
[0043] 1) Measure 16ml of ethylene glycol solution.
[0044] 2) Dissolve 404 mg of ferric nitrate nonahydrate, 99 mg of manganese chloride tetrahydrate and 120 mg of urea in the solution of step 1), and ultrasonically make the solution uniform.
[0045] 3) Transfer the solution obtained in 2) into a polytetrafluoroethylene liner with a volume of 18 ml.
[0046] 4) Put the inner lining in the steel kettle, screw it tightly and put it into the oven. Constant temperature at 200°C for 12h.
[0047] 5) After the reaction is completed, the reaction kettle is cooled down to room temperature with the oven, the solution in the polytetrafluoroethylene lining is poured out, and centrifuged to obtain a solid product, which is washed several times with ethanol and water in turn to obtain the target product.
[0048] Depend on Figure 4 It can be seen that the particle size of the obtained spherical manganese ferrite nanoclusters is about 200 nm. The size of the primary particles is abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com