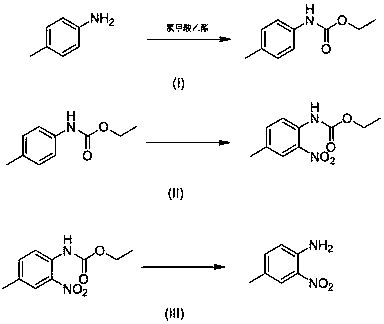
4-methyl-2-nitroaniline synthesis method
A technology of nitroaniline and methylaniline, which is applied in the field of clean preparation of organic chemicals, can solve the problems of high requirements for equipment and synthesis process conditions, strict process control requirements, and large environmental damage, so as to facilitate industrial production, The effect of clean reaction process and simple post-reaction treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Simply condense 4-methylaniline with ethyl chloroformate for amino protection to generate N-(p-toluene) ethyl carbamate, and then add 0.10 N-(p-toluene) ethyl carbamate to a 35.0 mL pressure-resistant tube mmol, 0.01 mmol of copper sulfate, 0.60 mmol of tert-butyl nitrite, 2 mL of 1,4-dioxane, using air as oxidant, placed in an oil bath and heated to 90 °C; TLC monitored the reaction (V 乙酸乙酯 : V 石油醚 = 1:10); after reacting for 6 h, extract the organic phase with ethyl acetate, combine the organic phases, wash with water (3×10 mL), dry over anhydrous sodium sulfate, filter with suction, obtain the crude product after vacuum distillation, use Ethyl acetate:petroleum ether=1:20 mixed organic solvent was the eluent, purified with silica gel column, and then hydrolyzed to obtain the target product; the final yield was 70%. 1 H NMR (300 MHz, CDCl 3 ) δ 9.71 (s, 1H), 8.44 (d, J = 8.7 Hz, 1H), 8.00 (s, 1H), 7.45 (d, J = 9.9 Hz, 1H), 4.26 (q, J = 7.1 Hz, 2H), 2.38 (s,3...
Embodiment 2
[0026] In a 35.0 mL pressure-resistant tube, add 0.10 mmol of N-(p-toluene) ethyl carbamate, 0.01 mmol of copper chloride, 0.60 mmol of tert-butyl nitrite, 2 mL of 1,4-dioxane, and use air as the oxidant , placed in an oil bath, the reaction was heated up to 90°C; TLC monitored the reaction (V 乙酸乙酯 :V 石油醚 = 1:10); after reacting for 6 h, extract the organic phase with ethyl acetate, combine the organic phases, wash with water (3 × 10 mL), dry over anhydrous sodium sulfate, filter with suction, obtain the crude product after distillation under reduced pressure, use Ethyl acetate:petroleum ether=1:20 mixed organic solvent was used as eluent, purified by silica gel column, and then hydrolyzed to obtain the target product; the final yield was 79%.
Embodiment 3
[0028] In a 35.0 mL pressure-resistant tube, add 0.10 mmol of N-(p-toluene) ethyl carbamate, 0.01 mmol of copper nitrate, 0.60 mmol of tert-butyl nitrite, 2 mL of 1,4-dioxane, and use air as the oxidant. Place the reaction in an oil bath and heat up to 90°C; TLC monitors the reaction (V 乙酸乙酯 :V 石油醚 = 1:10); after reacting for 6 h, extract the organic phase with ethyl acetate, combine the organic phases, wash with water (3 × 10 mL), dry over anhydrous sodium sulfate, filter with suction, obtain the crude product after distillation under reduced pressure, use Ethyl acetate:petroleum ether=1:20 mixed organic solvent was used as eluent, purified by silica gel column, and then hydrolyzed to obtain the target product; the final yield was 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com