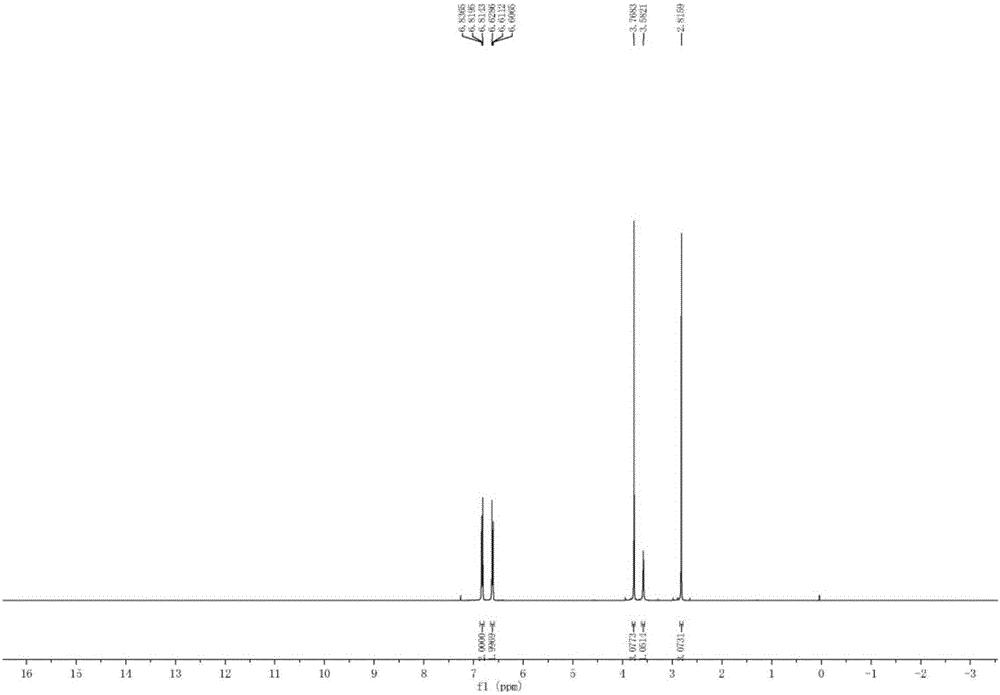
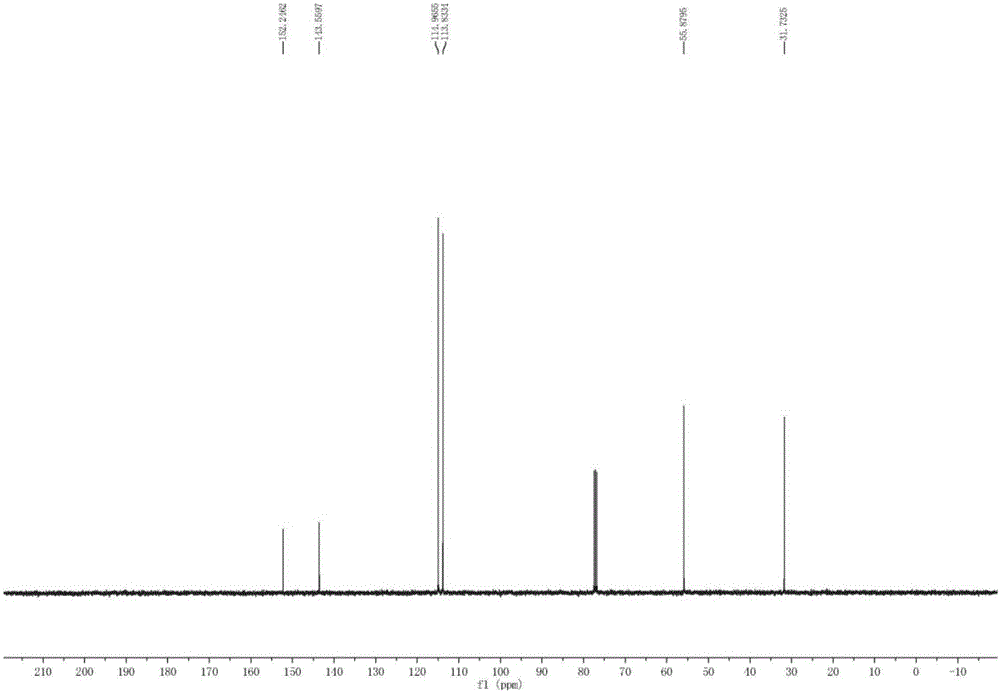
Preparation method of N-methyl-4-methoxyaniline
A technology of methoxyaniline and methyl, which is applied in the field of preparing N-methyl-4-methoxyaniline, can solve problems such as difficult industrial production, unfavorable environmental protection treatment, catalyst poisoning, etc., and achieve good product quality and improved The utilization rate of raw materials and the effect of reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The concrete steps of the method for described preparation N-methyl-4-methoxyaniline are:
[0035] (1) Synthesis of N-methyl-4-methoxyaniline
[0036] a. Put 100kg of p-aminoanisole, 500Kg (5w / w) of N, N-dimethylformamide, 26.8kg (1.1eq) of paraformaldehyde and 15kg of Raney nickel into the autoclave. Nitrogen was replaced 3 times, and then replaced with hydrogen 3 times. After the replacement, the system began to gradually heat up to 60 ° C, and hydrogen gas was started to control the reaction temperature at 60 ° C. The reaction pressure was controlled at 1.5 Mpa, and the reaction time was 8 hours until the system pressure no longer change.
[0037] b. When the reaction pressure is basically unchanged, close the hydrogen valve, and continue the reaction at this temperature and pressure for 1 hour. If the pressure remains unchanged, take a sample of the reaction solution and calibrate N-methyl-4-methoxy Aniline content, when the main content reaches more than 70%, it ...
Embodiment 2
[0048] The concrete steps of the method for described preparation N-methyl-4-methoxyaniline are:
[0049] (1) Synthesis of N-methyl-4-methoxyaniline
[0050] a. Put 100kg of p-aminoanisole, 200Kg (2w / w) of N, N-dimethylformamide, 17.07kg (0.7eq) of paraformaldehyde and 10kg of catalyst Raney nickel into the reaction kettle. Nitrogen was replaced 3 times, and then replaced with hydrogen 3 times. After the replacement, the system began to heat up gradually, and hydrogen gas was started. The reaction temperature was controlled at 80°C, the reaction pressure was controlled at 1.0Mpa, and the reaction time was controlled for 8h until the system pressure did not change any more. until.
[0051] b. When the reaction pressure is basically unchanged, close the hydrogen valve, and continue the reaction at this temperature and pressure for 1 hour. If the pressure remains unchanged, take a sample of the reaction solution and calibrate N-methyl-4-methoxy Aniline content, when the main co...
Embodiment 3
[0056] The concrete steps of the method for described preparation N-methyl-4-methoxyaniline are:
[0057] 1) Synthesis of N-methyl-4-methoxyaniline
[0058] a. Put 100kg of p-aminoanisole, 400Kg (4w / w) of N, N-dimethylformamide, 21.9kg (0.9eq) of paraformaldehyde and 2kg of catalyst Raney nickel into the reaction kettle. Nitrogen was replaced 3 times, and then replaced with hydrogen 3 times. After the replacement, the system began to gradually heat up, and hydrogen was started. The reaction temperature was controlled at 120°C, the reaction pressure was controlled at 0.6Mpa, and the reaction time was controlled for 8 hours until the system pressure did not change any more. until.
[0059] b. When the reaction pressure is basically unchanged, close the hydrogen valve, and continue the reaction at this temperature and pressure for 1 hour. If the pressure remains unchanged, take a sample of the reaction solution and calibrate N-methyl-4-methoxy Aniline content, when the main con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com